Meeting Summary
Ruxolitinib cream was approved in Europe for the treatment of non-segmental vitiligo with facial involvement in adults and adolescents (aged ≥12 years) in 2023. Speaking at an Expert Hub session at the European Academy of Dermatology and Venereology (EADV) 2025 Congress, Alexander Zink, Professor, Department of Dermatology and Allergy, Technical University of Munich, Germany; and Angelo Marzano, Professor of Dermatology and Head of Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, discussed how the introduction of ruxolitinib cream has shaped the management of vitiligo in the 2 years since its approval in this indication, based on insights from their own clinical experience. They described positive outcomes in patients who had used ruxolitinib cream, and discussed its practical use in real-world settings, including stopping and restarting treatment. Recent data on the efficacy of ruxolitinib cream in vitiligo with genital involvement, and when used in combination with phototherapy, were also presented.
Latest Clinical Findings for Ruxolitinib Cream in Vitiligo
Zink opened the session by recapping key efficacy data for ruxolitinib cream in non-segmental vitiligo from the TRuE-V1/2 registration trials,1 which demonstrated significant improvements in Vitiligo Area Scoring Index (VASI) scores, with approximately half of patients achieving ≥75% improvement in facial VASI score (F-VASI75) at Week 52.2 Repigmentation was observed across body regions,3 and half of patients achieved ≥50% improvement in total VASI score (T-VASI50) at Week 52.2
Vitiligo with Genital Involvement
Zink went on to present data from a recent Phase 2 study in patients with vitiligo involving the genital region (NCT05750823), which he described as an area of unmet need. The efficacy of ruxolitinib cream in genital regions was similar to total body efficacy, with an approximately 30% reduction in both genital VASI and T-VASI scores and an almost 20% reduction in genital body surface area involvement observed at Week 48. Over 20% of patients rated their genital vitiligo as a lot less or no longer noticeable.4
Ruxolitinib in Combination with Narrow-band UVB Phototherapy
The need for phototherapy alongside topical treatment for vitiligo is debated. Its role was explored in a recent Phase 2 open-label study (NCT03099304), in which patients who did not achieve T-VASI25 during 12 weeks of treatment with ruxolitinib cream alone were additionally treated with narrow-band UVB phototherapy three times per week for a further 36 weeks. Phototherapy appeared to accelerate the achievement of F-VASI and T-VASI responses in patients who did not respond quickly to ruxolitinib monotherapy.5
Long-Term Management of Vitiligo
The question of whether patients can stop treatment once they achieve satisfactory repigmentation was addressed in a long-term extension to TRuE-V1/2. A cohort of patients with an F-VASI90 response at Week 52 was randomised to continuation or withdrawal of ruxolitinib. Among patients who stopped treatment (n=56), 39% maintained response at >F-VASI75 to Week 104, but 29% relapsed (<F-VASI75) within a year of stopping (compared with 69% and 15%, respectively, among patients who continued treatment).6 Patients who relapsed could resume ruxolitinib as rescue treatment; 75% of these patients regained F-VASI75 after a median of 12 weeks’ retreatment, and approximately 70% regained F-VASI90 after a median of 15 weeks.6 In real-world practice, patients may stop and restart treatment; these data support retreatment as an effective approach.
Real-World Experience with Ruxolitinib Cream for Vitiligo: Insights from Clinical Practice in Germany and Italy
The efficacy of ruxolitinib cream demonstrated in clinical trials is reflected in the real-world experience of patients with vitiligo, illustrated in patient cases that Zink and Marzano shared from their own clinical practice.
Zink described a series of patients with vitiligo with facial involvement and confirmed that all had responded to ruxolitinib treatment.
He described some as ‘super-responders’, who achieved complete or near complete repigmentation within a few weeks (Figure 1), while others (typically those with fairer skin) were later responders. One patient had relapsed during a treatment break, but regained a good response on retreatment. Another patient had improvement in vitiligo-associated leukotrichia in his beard, as well as skin repigmentation. Zink also presented an example of a female patient with genital involvement who had a gradual response and achieved complete repigmentation after approximately 1 year of treatment (Figure 2).
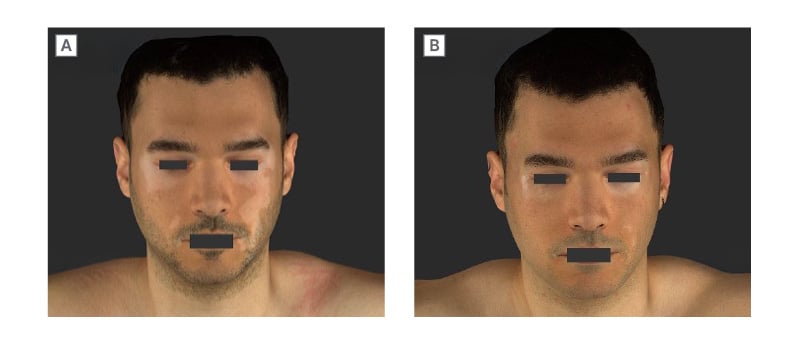
Figure 1: Patient case: rapid improvement in facial vitiligo with ruxolitinib cream.
Male (27) with vitiligo diagnosed in summer 2022. A) He had prior treatment with a topical calcineurin inhibitor, and commenced ruxolitinib cream the following year (baseline). B) Re-assessment after 6 weeks showed near-complete repigmentation of the face.
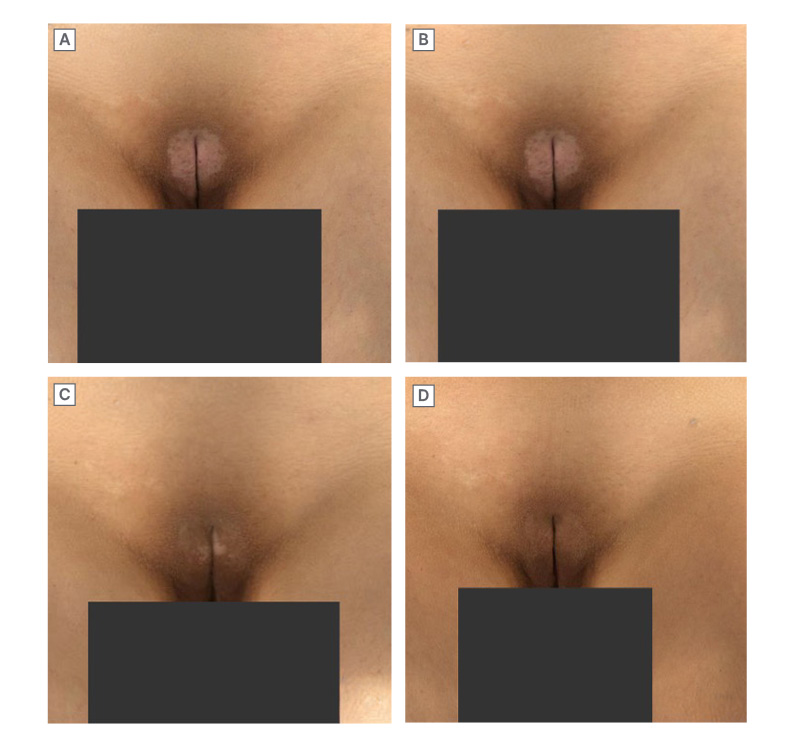
Figure 2: Patient case: resolution of genital vitiligo with ruxolitinib cream.
Female (24) with a history of vitiligo for approximately 2 years who had prior treatment with a topical calcineurin inhibitor. She commenced ruxolitinib cream monotherapy in September 2023
(A; baseline). Response was limited for approximately 5 months of treatment (B). Substantial response was seen at approximately 8 months (C), with complete repigmentation achieved after approximately 11 months (D).
Concurring that time to response varies between patients, Marzano highlighted the importance of managing expectations to encourage treatment adherence in patients who may require prolonged treatment to achieve a satisfactory response. He recommended continuing treatment for at least a year in these patients to ensure both adequate treatment duration and that treatment continues through the summer season, as patients may benefit from the addition of ‘natural phototherapy’.
Marzano outlined outcomes in patients participating in a trial running in his own clinic. Results to date suggest efficacy in line with that observed in the TRuE-V1/2 studies (unpublished data), and patient-reported outcomes reveal very good patient perception of improvement. He showed examples of patients who had achieved repigmentation of the face and other body areas, including a young female patient who never wore skirts/dresses because she wanted to hide vitiligo lesions on her knees. After 1 year of ruxolitinib treatment, she had a meaningful response (Figure 3) and no longer felt restricted in her choice of clothes, which had a positive impact on her mental health. Zink also described a patient who had grown his hair long to hide vitiligo lesions on his forehead but was able to cut his hair after achieving repigmentation with ruxolitinib cream. Marzano highlighted the value of talking to patients about how vitiligo impacts their lives to identify high-impact sites and support shared decision-making regarding treatment priorities.
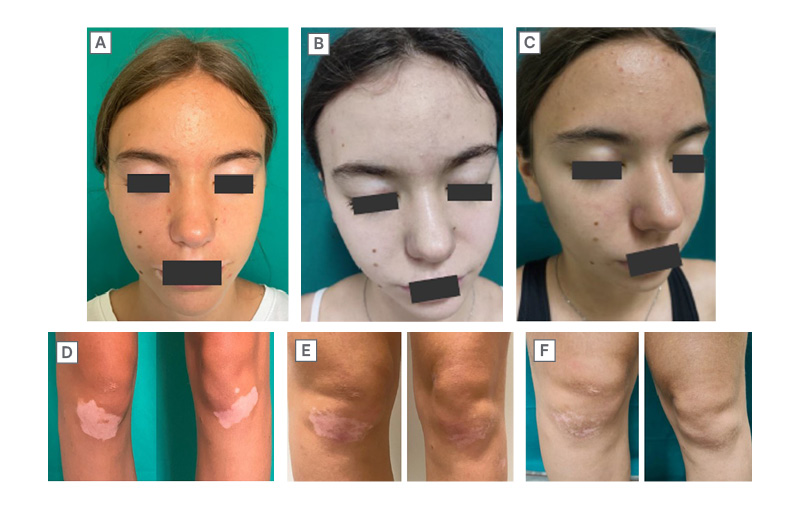
Figure 3: Patient case: meaningful response of facial and knee lesions to ruxolitinib cream.
A and D) Baseline; Facial-VESplus: 0.3375; Total-VESplus: 0.76375; CDLQI: 3. B and E) 24 weeks; Facial-VESplus: 0.0785; Total-VESplus: 0.546; CDLQI: 0. C and F) 52 weeks; Facial-VESplus: 0.0785; Total-VESplus: 0.536; CDLQI: 0.
Female (15) with a 4-year history of non-segmental vitiligo. She was treated with ruxolitinib cream twice daily on the face and knees as monotherapy. A response was observed by Week 24, with further improvement of knee lesions by Week 52. Patient-reported quality of life was also substantially improved.
CDLQI: Children’s Dermatology Life Quality Index; VESplus: Vitiligo Extent Score with perifollicular scale.
Both experts concurred that the safety/tolerability profile of ruxolitinib cream was excellent. Zink stated that he had never had a patient discontinue ruxolitinib cream due to local reactions.
Conclusions and Future Perspectives
More than 2 years’ experience with ruxolitinib cream in real-world clinical settings in Europe shows that the efficacy demonstrated in clinical trials is reflected in real-world effectiveness, and produces meaningful improvements for patients with vitiligo. Recent data support a role for ruxolitinib in patients with genital involvement and suggest a possible role for narrow-band UVB phototherapy to accelerate responses. Retreatment has proven effective in patients who relapse after stopping treatment, supporting the use of a stop–start strategy. Marzano highlighted a need for consensus on an optimal post-repigmentation maintenance schedule.
EU/RUXO/NP/0008
October 2025