Meeting Summary
Dr Robert welcomed the delegates to the symposium and outlined the agenda for the meeting, before presenting the first session on understanding cutaneous squamous cell carcinoma (CSCC). The rising incidence of CSCC presents a major challenge for healthcare systems and the frequency and impact of progression to advanced disease is underestimated. The diverse range and potential complications of CSCC lesions require a multidisciplinary approach, in which dermatologists play an important role. The message that CSCC remains a disease of high unmet need was echoed by Dr Peris, who presented on existing treatment approaches for the management of advanced CSCC. In the absence of an established management pathway for patients with locally advanced or metastatic disease, clinicians must rely on limited or anecdotal evidence to inform treatment decisions. Conventional chemotherapy and targeted therapies produce variable responses that are often short-lived, demonstrating a need for more effective and tolerable systemic treatments. Guidelines recognise these limitations and do not make any firm recommendations for the treatment of advanced CSCC. The importance of a multidisciplinary approach was underlined by Dr Migden’s presentation on future novel therapeutic strategies in CSCC. Immunotherapy is an exciting frontier that is becoming increasingly relevant to many specialists, including dermatologists, with several ongoing trials of immune checkpoint inhibitors in patients with advanced CSCC. A strong rationale exists for immunotherapy in these patients and the current evidence base supports the use of immune checkpoint blockade as an alternative to cytotoxic chemotherapy and targeted agents. Dr Migden concluded the symposium with an interactive presentation of five case studies of successful checkpoint inhibitor treatment of locally advanced CSCC, emphasising the key role of dermatologists in a multidisciplinary team approach.
Understanding Cutaneous Squamous Cell Carcinoma
Doctor Caroline Robert
CSCC is characterised by the proliferation of atypical keratinocytes in the skin. Diagnosis is usually straightforward, based on pathological findings of mitoses, differentiation with keratinisation, and, in some cases, invasion into deeper layers of tissue. Although the majority of patients present with small, localised lesions that are resectable, some may present with more advanced lesions and high-risk factors that can be a cause for concern in terms of prognosis.
Perhaps the greatest concern is the dramatic increase in incidence of CSCC worldwide, which shows few signs of halting. In one USA population-based study, the incidence of CSCC increased by 263% from 1976–1984 to 2000–2010, disproportionately higher than the corresponding increase in basal cell carcinoma (BCC).1 CSCC is now ranked the second most frequent skin cancer overall after BCC,2 with a geographic distribution that clearly demonstrates an association with cumulative sun exposure, a major risk factor for both CSCC and BCC.2,3
CSCC predominantly affects men, with a male:female ratio of 3:1, and incidence increases with age (average age of onset is in the mid-60s).2 In an Australian study from 2002, the incidence of CSCC was as high as 499 cases per 100,000 men, whereas European reports show a much lower incidence rate of up to 96 cases per 100,000 men.2 However, there is little doubt that the true incidence of CSCC is higher than reported, due in part to a scarcity of well-conducted tumour registries that include CSCC. It is also speculated that the mortality rate of CSCC is underestimated. Death can result from local or regional lymph node infiltration rather than due to distant metastases. In the USA, an estimated 4,000–8,000 people die annually from CSCC, based on a study conducted in 2012.2
Additional risk factors associated with CSCC include immunosuppressive status, chronic wounds and ulcers, oncogenic viral infections (e.g., human papillomavirus), smoking, and environmental exposure to ionising radiation and chemical carcinogens.2 Genetic predisposition to CSCC has been noted in patients with familial cancer syndromes, such as xeroderma pigmentosum, and hereditary skin disorders, such as albinism.4 The increased frequency of CSCC with immunosuppression is clearly evident in transplant recipients.2,5 In this population, CSCC is more frequent than BCC and often takes an aggressive form. Risk for CSCC increases with time following transplantation (10% of patients develop CSCC within 10 years) and is often related to the intensity of immunosuppression.5
A distinguishing feature of CSCC is that tumours frequently occur on precancerous lesions, most commonly in areas exposed to mutagenic ultraviolet (UV) radiation (both natural and artificial sunlight).2 Typical presentations include patients with multiple actinic keratoses, scalp lesions, and lesions erupting from old scar tissue (Figure 1). In some cases, CSCC may be highly differentiated with keratin synthesis, giving rise to a skin ‘horn’. Unlike BCC, CSCC can involve the mucosa and semi-mucosa (e.g., on the lips). Other histopathological subtypes include subungual and verrucous lesions, as well as CSCC in situ (Bowen’s disease).
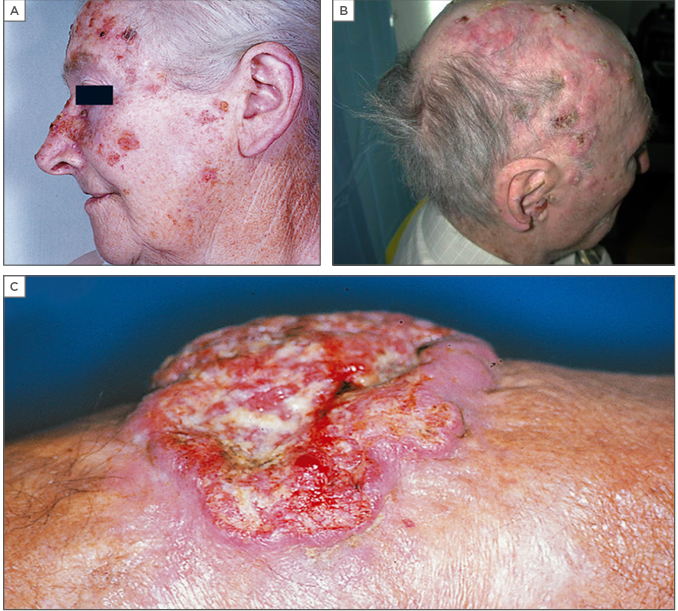
Figure 1: Common presentations of cutaneous squamous cell carcinoma.
(A) Cutaneous squamous cell carcinoma occurring in a patient with multiple actinic keratoses; (B) Patient with multiple cutaneous squamous cell carcinoma scalp lesions; (C) Patient with cutaneous squamous cell carcinoma occurring on a very old burning scar.
Images are from Dr Robert’s personal files and permission has been obtained to use them.
Many dermatologists are experienced in determining the vascular characteristics of CSCC lesions. Techniques such as dermoscopic semiology are useful to identify pigmented variants and other atypical presentations (e.g., hairpin/looped and serpentine vessels, which are characteristic of invasive CSCC) that can help to make an early diagnosis.2 In general, timely diagnosis of CSCC can be challenging as patients often do not present until the disease is advanced. For example, even educated patients in countries with good access to healthcare sometimes may not seek immediate care, because the tumour is not initially bothersome, or they are vulnerable and afraid.
Although no standard definition of high-risk CSCC currently exists, certain patient and tumour characteristics are known to be associated with local recurrence and metastatic disease. Pejorative factors include treatment-refractory and aggressive tumours, and locations, such as the ears and lips, that are more prone to be associated with lymph node metastases. High-risk histological factors include poor differentiation, neurotropism, and desmoplasia.2
In summary, the frequency and rising incidence of CSCC represents a major public health concern. Many different physician specialities, including dermatologists, are faced with a patient population that is vulnerable because of age; therefore, prevention of CSCC is important in all patients.5 Strategies include minimising cumulative exposure to both UVA and UVB. Although most cases of local CSCC are curable by surgical resection, it is not uncommon for tumours to progress to locally advanced or metastatic disease necessitating further treatment.2
Existing Treatment Approaches for the Management of Advanced Cutaneous Squamous Cell Carcinoma
Doctor Ketty Peris
Currently there is no clear treatment pathway once CSCC progresses to locally advanced or metastatic disease. Patients with unresectable or recurrent tumours have limited therapeutic options and prognosis is generally poor. Radiotherapy may be offered, usually with palliative rather than curative intent, and has demonstrated improved tumour control in both the primary and adjuvant settings.6 Potentially curative options include electrochemotherapy (ECT), chemotherapy, targeted therapy, and immunotherapy. As yet, none of these therapies are approved for advanced CSCC. Dermatologists and other clinicians must rely on limited or anecdotal evidence of efficacy and tolerability of these methods and the agents used.
ECT may be used for primary CSCC and cutaneous metastases of all histological subtypes.7 Treatment involves intravenous or intralesional injection of a cytotoxic agent (typically bleomycin or cisplatin), followed by insertion of electrodes that can deliver a series of electric pulses directly to the tumour and immediately to the surrounding tissues. The primary effect of ECT is to permeabilise cells within the exposed tissues, enabling increased intracellular transport of the cytotoxic agent. It also has a vasoconstrictive effect, enhancing tumour cell destruction by trapping the cytotoxic agent within the tumour vascular network and disrupting blood flow. Reported response rates to ECT are variable and dependent on tumour size.7,8 Notably, a small number of complete responses have been reported after a single cycle in patients with locally advanced CSCC >5 cm in diameter, or invading deeper tissues.9
The use of conventional chemotherapy in advanced CSCC has not been rigorously explored in randomised clinical trials, but rather through a number of retrospective studies and case series. The current evidence base is limited in terms of patient numbers and by wide heterogeneity in treatment regimens and clinical endpoints.10,11 Reported overall response rates (ORR) with monotherapy range from 17–78%, though these responses are typically short-lived and overall survival is not substantially extended. Anecdotal evidence suggests that combination therapy may be more effective than single agents in patients with advanced disease.10 A non-randomised Phase II trial investigating cisplatin in combination with INF-α and retinoic acid reported ORR of 67% (8 of 12 patients) in locally advanced CSCC and 17% (4 of 23 patients) in metastatic disease. Complete responses were observed in six patients with a median duration of 35.4 months.12 Complete responses have also been reported in studies of other cisplatin-based combinations, albeit in small numbers of patients.10,13
The identification of driver mutations that are important for the progression of CSCC has paved the way for targeted therapies. Epidermal growth factor receptor (EGFR) inhibitors are the most widely studied, following the discovery that EGFR is overexpressed in undifferentiated, proliferating keratinocytes in the basal epidermis as well as in outer layers of hair follicles and in sebaceous and eccrine sweat glands.14,15 EGFR blockade in CSCC is believed to reduce proliferation and induce premature differentiation of tumour cells. However, clinical trial experience is limited. The largest study to date was a prospective Phase II study of cetuximab in 36 chemotherapy-naïve patients with EGFR+ locally advanced or metastatic CSCC, which demonstrated a disease control rate of 69% at Week 6, with two complete and eight partial responses.16 The use of cetuximab alone or in combination with chemotherapy is further supported by studies with a low level of evidence, mainly case series and case reports.17-19 A systematic review, including 69 cases of metastatic CSCC with large primary lesions, concluded that cetuximab produced longer disease-free survival than cisplatin (25.0 versus 14.6 months).11 The evidence for erlotinib and gefitinib is similarly limited in advanced CSCC, with only modest activity reported.20,21
The lack of good quality evidence available for systemic therapies in advanced CSCC is reflected in current guidelines and treatment recommendations.6,22,23 The variable response rates and lack of consistent reporting in studies of cytotoxic chemotherapy and EGFR inhibitors preclude any firm conclusions about relative effectiveness. Treatment decisions must, therefore, be guided by the needs of individual patients, particularly with respect to acceptable tolerability. For example, ECT may not be acceptable for patients with large lesions due to reports of pain and the risk for massive tumour necrosis.8 Tolerability issues associated with cytotoxic chemotherapy are well documented and frequently limit the use of these agents, particularly in older patients and those with poor performance status.24 Common side effects of cisplatin include nausea and vomiting, leukocytopenia, and anaemia.11 Dose-cumulative nephrotoxicity and peripheral neuropathy are also important concerns.25 Although EGFR inhibitors are generally better tolerated than conventional chemotherapy, the majority of patients develop dose-dependent cutaneous toxicities.26-30 The most common toxicity, occurring in 45–100% of patients, is a papulopustular/acneiform rash that usually appears within 1–2 weeks of initiating therapy and causes pruritis, burning, and pain.29 As these toxicities often affect aesthetically sensitive areas, they can impact a patient’s quality of life and may result in treatment discontinuation.
In summary, there are currently no approved systemic therapies for locally advanced or metastatic CSCC and much of the existing data are derived from small, mostly retrospective studies. Responses rates are variable, typically with short-term responses that are often unsustainable due to drug toxicity. Guidelines have so far not provided firm recommendations for systemic treatment of either locally advanced or metastatic disease. Until further data-driven recommendations can be made, the suggestion is that individual cases of advanced CSCC should be evaluated and managed in the context of a multidisciplinary tumour board.6,22,23 Participation in a clinical trial should also be recommended.22
Future Novel Therapeutic Strategies, Including Checkpoint Blockade, for Advanced Cutaneous Squamous Cell Carcinoma
Doctor Michael Migden
Cancer immunotherapy is not a new concept, but renewed interest in the field was motivated by the discovery of immune checkpoint molecules, starting with programmed cell death protein 1 (PD-1) and its ligand (PD-L1) in the early 1990s.31 Several inhibitors of these molecules have since become established anticancer therapies, approved for a range of solid tumours and haematological malignancies,32 and represent an exciting new frontier in dermato-oncology. Activation of the PD-1/PD-L1 axis is presumed to serve as a mechanism for tumour evasion of immunosurveillance through suppression of T cell-mediated tumour destruction. Antibodies targeting PD-1 or PD-L1 work by preventing binding of PD-1 to PD-L1, releasing the brakes on the immune system and helping to restore T cell function (Figure 2).32-34
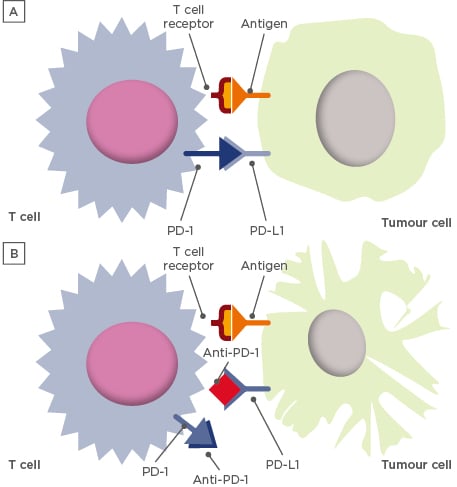
Figure 2: Simplified schematic of the function of the programmed cell death protein 1/programmed death ligand-1 axis in immunosurveillance.32,33
Activation of PD-1/PD-L1 pathway suppresses T cell-mediated tumour destruction, providing a rationale for anti-PD-1/PD-L1 immunotherapies. (A) Binding of PD-1 to PD-L1 leads to downregulation of T cell-mediated tumour destruction;34 (B) Blocking the interaction with anti-PD-1/PD-L1 agents helps to restore T cell function for an antitumour response.
PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1.
A strong rationale exists for evaluating checkpoint inhibitors in advanced CSCC. Firstly, CSCC has the highest mutational burden of any tumour in the Cancer Genome Atlas. Median somatic mutation frequency per megabase of DNA for CSCC is around 4.6-times higher than for melanoma (an approved indication for some checkpoint inhibitors). Higher tumour mutational burden is believed to contribute to increased neoantigen production, which may increase tumour antigenicity and make the tumour more susceptible to immunotherapy.35 Secondly, an immune component of CSCC is implied by the increased risk of cutaneous lesions with chronic immunosuppression, especially in transplanted patients.36 Thirdly, PD-L1 protein expression (a clinically relevant biomarker for response in other tumour types) correlates with metastatic risk in CSCC. Importantly, it is the presence rather than the extent of PD-L1 expression that predicts metastatic risk. In an immunohistochemical review of 45 CSCC lesions, low PD-L1 expression (defined as a tumour proportion score of 1–49%) was observed in the majority of high-risk and metastatic tumours.37
Potential candidates for immunotherapy for advanced CSCC are those who have failed multiple previous surgeries, or who are not surgical candidates due to potential morbidity, disfigurement, or low confidence for obtaining clear margins, and are not candidates for radiotherapy. PD-1 inhibitors currently being investigated in Phase II clinical trials include cemiplimab38 and pembrolizumab39 as monotherapy, and nivolumab in combination with the experimental cancer vaccine talimogene laherparepvec (T-VEC).40 The PD-L1 inhibitor, atezolizumab, is also in Phase II development, both as monotherapy and in combination with the mitogen-activated protein kinase kinase (MEK) inhibitor, cobimetinib.41 A retrospective analysis of 18 patients with aggressive CSCC treated with a PD-1 inhibitor (nivolumab or pembrolizumab) reported an ORR of 77.8% (14 patients), with 4 complete responses. Mean time to observed response was relatively short (2.7 months), with a median duration of response of 12 months.42 A case report of two major responses with PD-1 inhibitors has also been published.43
Checkpoint inhibitors are generally well tolerated, but immune-related adverse events (irAE) are possible, as with any other immunotherapy. irAE are distinct inflammatory toxicities caused by non-specific activation of the immune system that can affect any organ, most commonly the gastrointestinal tract, endocrine glands, skin, and liver.44,45 Events are typically low-grade and most develop in the first few weeks or months of treatment, though they may present later and can fluctuate over time.46 irAE are generally manageable with prompt identification and treatment according to established management pathways,47-49 with emphasis on collaboration with multidisciplinary partners across the clinical spectrum.50 Notably, the frequency of Grade ≥3 irAE appears to be 3–4 times lower with antibodies targeting PD-1/PD-L1 than with antibodies targeting cytotoxic T lymphocyte-associated protein 4 (CTLA-4, an immune checkpoint molecule), when used as single agents.47 In some cases, the development of irAE may be a sign of increased immune activity and could potentially predict a durable response. This association has been reported in patients with melanoma treated with the CTLA-4 inhibitor, ipilimumab, but the underlying mechanism remains unclear.46
Although uncommon, pseudoprogression with immunotherapy is an important concept, and immune-related response criteria have been developed to avoid misclassification as true disease progression.51 Pseudoprogression is characterised by inflammatory changes in the tumour (e.g., increase in erythema, oedema, or tumour size), likely due to the infiltration of the tumour by activated T cells.51,52 In other words, pseudoprogression is a sign of active treatment rather than true disease progression and may in fact be a signal for improved patient outcomes.53 Indeed, tumour-infiltrating lymphocytes may be used as a biomarker of response, alongside PD-L1 expression as previously mentioned. Additional biomarkers are being investigated in CSCC, including, but not limited to, CD133 (a cancer stem cell protein), Serpin A1 (a serine peptidase inhibitor), and EphB2 (an erythropoietin-producing hepatocellular receptor).54 Concerns have been raised about hyperprogression in patients with solid tumours treated with PD-1/PD-L1 inhibitors, particularly in elderly patients55 and those with high metastatic burden or poor prognosis.56 However, it is too early to say whether hyperprogression occurs in CSCC.
Immune checkpoint blockade is becoming increasingly relevant to dermatologists, particularly in Europe where dermato-oncology is already an established speciality. The strong rationale for immunotherapy in patients with locally advanced and metastatic CSCC is supported by the limited evidence base, and ongoing trials evaluating PD-1 and PD-L1 inhibitors in these patients will provide further insight into the use of these agents. In conclusion, Dr Migden reiterated an earlier point that a multidisciplinary approach is essential for appropriate management and follow-up of CSCC, and that dermatology specialists can have valuable input in every treatment decision.
Case Study Presentations
Doctor Michael Migden
Dr Migden closed the presentation sessions by sharing five case studies of patients with locally advanced CSCC successfully treated with a checkpoint inhibitor. Two cases were of particular interest. The first was a male patient with no clear surgical target due to in-transit tumour field with a history of Mohs surgery and additional tumour resection by a head and neck surgeon. Within months of latissmus flap surgery, recurrences were noted at five locations on the margins of the free flap. In consecutive polls, the audience voted for multidisciplinary evaluation of the patient (including dermato-oncology, medical, and radiation oncology specialists, and a head and neck surgeon) and consideration of a clinical trial, and that a further large resection should be avoided. Biopsies of lesion sites were in-transit tumour-negative after 7 months of treatment with a checkpoint inhibitor, and lesions resolved with an additional 1 month of treatment. The patient was subsequently able to stop treatment with no evidence of disease recurrence.
The second case was a male with a massive, deeply eroded premaxillary tumour with an orocutaneous fistula causing difficulty eating due to pain and a large volume of saliva leak. The case was referred to Dr Migden by a head and neck surgeon who was concerned that surgery would be functionally debilitating and had doubts that the fistula could be closed without surgery. An audience poll supported the view of the referring surgeon, with delegates voting that chemotherapy alone would be unlikely to lead to closure of the fistula. Remarkably, closure was achieved within 3 months of initiating checkpoint inhibitor therapy. The patient had substantially less pain, no saliva leak, and no further surgery was required.
In the discussion following the case presentations, Dr Migden confirmed that of the 17 patients with advanced CSCC treated in his centre with a checkpoint inhibitor, the majority had good disease control. A minority of patients reported irAE that were manageable.







