AIMS
Positive renal effects of vildagliptin have been shown by several experimental studies.1,2 Nonetheless, clinical evidence for its renoprotective potential is limited and predictive determinants are unknown.3 To assess the renal effects of vildagliptin and identify their clinical and laboratory predictors, 47 insulin-treated male and female Caucasians, aged 49–70 years, with satisfactorally controlled Type 2 diabetes mellitus (T2DM) and blood pressure (BP) were enrolled. Patients had no overt chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR]: ≥60 mL/min; and the absence of proteinuria), morbid obesity, severe vascular diabetic complications, non-diabetic renal impairment, liver failure, or systemic autoimmune disorders. Patients were randomised either to continue insulin therapy (control) or to receive vildagliptin in a daily dose of 50 mg added-on insulin (V+ group). At baseline and after 6 months of treatment, we assessed eGFR (with the CKD Epidemiology Collaboration creatinine equation) using serum creatinine (eGFRcr), cystatin C (eGFRcys), both (eGFRcr-cys), and creatinine-adjusted urinary markers of glomerular (urine albumin-to-creatinine ratio, collagen Type IV [uColIV]) and tubular injury (L-Type fatty acid binding protein [L-FABP]), as well as metabolic control parameters.
RESULTS
Forty-four patients completed the study. The groups were comparable on the basis of sex (42.9% male in control group and 39.1% in V+ group), age (median value control group: 60.0 years [25th percentile: 54.0; 75th percentile: 66.0] versus 62.0 years [58.0; 63.0] in the V+ group), and known T2DM duration (≥10.0 [6.0; 13.0] years versus 8.0 [7.0; 12.0] years, respectively) (p>0.05 for each). A2 category of CKD was detected in 47.6% of control patients and in 52.2% in the V+ group (p=0.76). Arterial hypertension was observed in 81.0% and 86.9% of patients in the control group and V+ group, respectively (р=0.59), and clinically manifested atherosclerosis in 52.4% and 47.8%, respectively (р=0.76). Renin-angiotensin- modulating therapy was received by 76.2% and 82.6% of patients, respectively (p=0.6).
As shown in Table 1, at baseline there were no significant differences in assessed laboratory parameters between the two groups. In the control group, no parameter changed significantly after 6 months of the treatment. Compared to the dynamics in the control group, patients from the V+ group demonstrated a significant decrease in glycated haemoglobin (HbA1c) and insulin requirement as well as the frequency of hypoglycaemia. Furthermore, a significant reduction in diastolic BP, serum cystatin C, and excretion of uColIV was documented in the V+ group, as well as an increase in eGFRcys and eGFRcr-cys. When we compared the repeated measurements of the study markers, only the changes in serum cystatin C and eGFRcys in the V+ group differed significantly compared to the control. Correlation analysis showed that neither changes of serum cystatin C, eGFRcys, and eGFRcr-cys, nor changes of uColIV in the V+ group, were significantly related to the dynamics of HbA1c (r: -0.31, 0.21, 0.19, and 0.13, respectively; p>0.05 each). We found an inverse association between the changes in systolic BP and eGFRcr-cys (β: -0.47; R2: 0.22; р=0.02), suggesting that haemodynamic mechanisms at least partially contribute to the renal action of vildagliptin. Stepwise regression analysis showed that lower levels of baseline eGFRcys were independent predictors of both eGFRcys and eGFRcr-cys increase (β: -0.61; R2: 0.37 and β: -0.45; R2: 0.20, respectively; p<0.05 each). Reduction of uColIV excretion was more pronounced in older patients (β: -0.74) with lower levels of diastolic BP (β: 0.57; R2: 0.46; p=0.002).
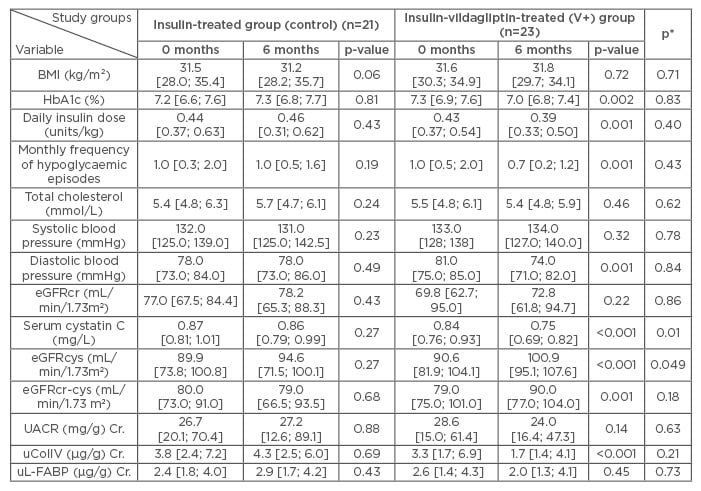
Table 1: Clinical and laboratory parameters of patients with T2DM in the studied groups at baseline and after 6 months of the treatment.
The median [25th percentile; 75th percentile] are shown. р-values at 0 months versus 6 months at the same group (the Wilcoxon signed-rank test). р*: comparisons between both measurements of studied groups (repeated measures in the general linear models). Nonsignificant differences between baseline values in the study groups (Mann-Whitney U-test) are not shown.
Cr: creatinine-adjusted; eGFRcr: estimated glomerular filtration rate using serum creatinine; eGFRcr-cys: estimated glomerular filtration rate using serum creatinine and cystatin C; eGFRcys: estimated glomerular filtration rate using cystatin C; HbA1c: glycated haemoglobin; T2DM: Type 2 diabetes mellitus; UACR: urine albumin-to-creatinine ratio; uColIV: urine collagen Type IV; uL-FABP: urinary L-Type fatty acid binding protein.
CONCLUSION
Vildagliptin administration to insulin-treated T2DM patients was associated with a reduction of the glomerular injury marker, uColIV excretion, along with an increase of eGFRcr-cys and eGFRcys, independent of glycaemic control. Older age and lower baseline values of diastolic BP were predictive of a better uColIV-response in the V+ group. Since eGFR improvement could result from temporary hyperfiltration,4 long-term studies of the renal effects of vildagliptin with isotope clearance eGFR measurement are required.








