Abstract
Introduction: Metabolic syndrome (MetS), also known as insulin resistance syndrome, is described as a cluster of cardiometabolic symptoms such as high blood pressure, elevated fasting glucose, or insulin resistance. MetS is one of the most serious public health problems being faced globally. The purpose of the current investigation was to determine its prevalence, as well as the relationship between blood adiponectin levels and the development of MetS.
Materials and Methods: This observational cross-sectional hospital-based study was performed in the Department of General Medicine, School of Medical Sciences and Research, Sharda Hospital, Greater Noida, Uttar Pradesh, India, from January 2019–June 2020. Sixty patients attending the medicine out- or inpatient department, who confirmed consent, and fit into the International Diabetes Federation (IDF) inclusion criteria for MetS, were recruited for this study. The final sample size for this study was found to be 60, with a prevalence of 10%. This is the reason the study’s precision decreased to ±7.6%, implying that the precision of the end result may vary by ±7.6%.
Results: Subjects without MetS were on average younger, had a lower BMI, and had a smaller waist circumference than those who had MetS, according to the findings. They also had lower blood pressure, pulse rate, and fasting plasma glucose levels than the people with MetS, and there were statistically significant variations in lipid profiles between those with and without MetS. In people who did not have MetS, the mean serum adiponectin concentration was 15.79±2.90 mg/mL, whereas the mean serum adiponectin concentration in people who did have MetS was 11.02±2.63 mg/mL (p<0.001). The levels of adiponectin were compared with the different components of MetS as defined by the IDF. The mean adiponectin concentrations in connection to the clinical characteristics of MetS are shown in Table 1 . The authors discovered that lower adiponectin levels were statistically significantly linked with the majority of the characteristics. In a multivariate analysis, the serum adiponectin content was found to be significantly inversely associated to systolic blood pressure (r=-0.262; p<0.050), BMI (r=-0.288; p<0.050), total cholesterol (r=-0.515; p<0.001), and low-density lipoprotein (r=-0.305; p<0.050) in the study participants.
Conclusion: In conclusion, the present results suggest that circulating levels of adiponectin are reduced in the presence of MetS.
Key Points
1. Metabolic syndrome (MetS) is an insulin resistance syndrome with cardiometabolic symptoms, including high blood pressure, elevated fasting glucose, or insulin resistance, and is one of the most serious public health problems being faced worldwide.2. This observational cross-sectional hospital-based study carried out in India investigates the relationship between adiponectin levels in MetS and other MetS components.
3. The association between adiponectin and high-density lipoprotein cholesterol, triglycerides, fasting blood sugar, BMI, and waist circumference could explain the reason why people with MetS have lower levels of adiponectin compared to the general population.
INTRODUCTION
Metabolic syndrome (MetS), also known as an insulin resistance syndrome, has been related to higher blood pressure outside of the normal range, insulin resistance, and increased fasting glucose levels.1 It is one of the world’s most important public health issues due to its growing prevalence in society and its impact on a large number of people.2 In today’s scenario, it is an alarming healthcare issue; however, if detected early enough, further complications can be avoided and quality of life improved. According to the World Health Organization (WHO), both developed and developing countries are seeing a growth in the number of cases of MetS. It affects anywhere from 15–40% of the population, with a higher prevalence rate in impoverished nations than in more developed ones.3 MetS is related to an increased risk of cancer, in addition to coronary heart disease, Type 2 diabetes (T2D), and other cardiometabolic disorders.4 MetS is a collection of risk factors for cardiovascular disease (CVD) that includes insulin resistance, obesity, hypertension, elevated triglycerides, and low levels of high-density lipoprotein (HDL).5
MetS is a powerful predictor of T2D, as people affected by the disease have a five to seven-fold greater chance of getting T2D. Those with MetS are also twice as likely to have CVD.6 The heterogeneous condition is caused by obesity, particularly visceral adiposity, and insulin resistance. It is a group of common clinical diseases that includes obesity, hypertension, insulin resistance, glucose intolerance, and dyslipidaemia.7 It has been suggested that visceral fat deposits are more implicated in the advancement of obesity-related disorders such MetS, T2D, and coronary artery disease, since they have a higher metabolic activity than subcutaneous fat deposits.8 Adiponectin is an adipocytokine, which means it regulates blood sugar and cholesterol levels, as well as the cardiovascular system.
The amount of adiponectin in people with obesity was shown to be lower, despite the fact that it is mostly generated by adipose tissue, and higher levels were related to a drop in overall body weight in patients with obesity, implying an inverse relationship between adiponectin and body weight. This could be explained by the fact that subjects who are overweight receive negative feedback.9
Adiponectin contributes to the pathogenesis of cardiac MetS through various mechanisms. It has a detrimental influence on blood glucose and free fatty acid levels, among other factors.10 To the authors’ delight, it appears that the connection between insulin resistance and adiponectin secretion runs both ways. Adiponectin improves insulin receptor sensitivity in peripheral tissues, affecting food intake, metabolic rate, and body weight.11 Insulin resistance was shown to be worsened, and increasing insulin resistance was linked to lower levels of the bioactive adiponectin in those with high insulin levels. Inflammation and oxidative stress, both of which are related to insulin resistance, decrease adiponectin levels.12
It has been proven that the hormone serum adiponectin has an inverse connection with insulin resistance and body fat mass in adults. People with T2D and those with coronary artery disease have abnormally low levels in their blood. As a result of this, adiponectin is commonly believed to promote insulin sensitivity and to contribute to cardiovascular protection.13 Lower than normal levels of the high molecular weight component of adiponectin in plasma, in particular, is a significant risk factor for the onset of MetS.14 Fatigue, excess weight, and abdominal obesity were the most common anomalies among people with MetS, especially in the abdominal area. Because of its connection to insulin resistance and low-grade chronic inflammation in the body, abdominal obesity is usually considered as the most significant underlying cause of MetS.15 The waist-to-hip ratio, commonly known as the waist-to-height ratio, is a better predictor of MetS than the BMI.16
IL-6 and other pro-inflammatory cytokines have been related to poor metabolic health in both people with and without obesity. Adipokines like adiponectin, on the other hand, have been linked to improved metabolic health in both groups.17 Increased IL-6 levels and reduced adiponectin have been related to MetS and related processes in the absence of disease.18 Because the MetS pandemic has been detected in a wide range of populations, it is urgently necessary to investigate the mechanism underlying the disease, as well as to search for robust and sensitive biomarkers that can be used as a diagnostic tool for the early detection and diagnosis of MetS, both in humans and in animals. Adiponectin and MetS are related in a range of ethnic groups, including White, Korean, and Japanese people. In the Indian population, the relationship between adiponectin levels and MetS criteria is yet unknown, as a limited number of Indian studies are available, including within the south of the country. To the authors’ knowledge, this is one of the first studies to be conducted in their region, evaluating the relationship between adiponectin levels in MetS and other MetS components. This is a huge oversight, given the high prevalence of MetS, diabetes, and CVD in this part of India. Adiponectin levels have been associated with an increased risk of cardiovascular mortality in patients with diabetes; however, this is assumed to be a response to microvascular issues rather than a separate risk factor. The authors wanted to see whether there was a connection between MetS and blood adiponectin levels in healthy people.
MATERIALS AND METHODS
This observational cross-sectional hospital-based study was performed in the Department of General Medicine, School of Medical Sciences and Research, Sharda Hospital, Greater Noida, Uttar Pradesh, India, between January 2019–June 2020. Sixty patients attending the medicine out- or inpatient department and fitting into the International Diabetes Federation (IDF) inclusion criteria for MetS were recruited for this study. The study’s final sample size was determined to be 60 individuals, with a 10% prevalence rate. This is the reason the study’s precision decreased to ±7.6%, implying that the precision of the end result may vary by ±7.6%. The study was conducted after taking approval from the institutional ethical committee and written informed consent from the participants.
IDF inclusion criteria are visceral obesity, defined as waist circumference ≥102 cm in males and ≥88 cm in females; fasting plasma glucose ≥100 mg/dL; systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg, or patient on antihypertensive treatment; and serum triglycerides ≥150 mg/dL or patient on lipid lowering treatment, and HDL-cholesterol (HDL-C) <40 mg/dL in males and <50 mg/dL in females.
After a written informed consent form had been obtained, detailed history of the presenting symptoms and their onset was recorded. Detailed histories of all the patients were obtained, including demographic details and age of the patient; and clinical details such as blood pressure, heart rate, lipid profile, BMI, and blood sugar level (fasting and prandial) were noted on patients’ proforma.
Analysers used enzyme digestion to measure blood glucose, total cholesterol (TC), HDL-C, and triglycerides in the fasting stage of the bloodstream. Dextran sulphate and magnesium chloride were used to precipitate non-HDL-C, and HDL-C was tested afterwards. Low-density lipoprotein (LDL)-cholesterol was estimated using Friedewald’s equation in blood samples with triglycerides below 400 mg/dL. The levels of adiponectin in 180 randomly selected sera were measured using an ELISA (Immuno Concept Pharmacueticals, Sacramento, California, USA) in this sub-study. The intra- and inter-assay coefficients of variation of adiponectin were 10% and 13%, respectively. The second sample was treated with ethylenediaminetetraacetic acid and centrifuged at 3,000 xg for 15 minutes before the adiponectin level was determined at -20 oC. Fasting blood glucose, serum uric acid, and lipid profile were all tested using ViTROS® FS 5.1 (Ortho Clinical Diagnostics, Raritan, New Jersey, USA). Colorimetric techniques were used to measure the results. In order to determine postprandial blood glucose levels on the VITROS 5.1 analyser, the authors collected samples 2 hours after the meal.
For the data analysis, SPSS Statistics version 23.0 (International Business Machines Corporation [IBM], Armonk, New York, USA) on Windows (Microsoft, Redmond, Washington, USA) was utilised, and Excel (Microsoft) was used to construct the database, as well as generate the graphs. To describe quantitative data with a normal distribution, the mean and standard deviation were utilised. Statistical difference between two groups was analysed using the student t-test. Data were used to assess adiponectin levels in patients with MetS. When p values of <0.05 were attained, statistical significance was considered to be achieved.
RESULTS
Subjects without MetS in this study were younger, had lower BMIs, and had smaller waist circumferences in comparison to individuals with MetS; however, there were significant differences in overall lipid profiles between those with and without MetS as well. Those without MetS also had lower blood pressure, pulse rates, and fasting plasma glucose levels than subjects with MetS. Subjects without MetS had a higher mean serum adiponectin concentration (15.79±2.90 mg/mL) than those with MetS (11.02±2.63 mg/mL), with statistical significant difference as p<0.050.
The levels of adiponectin were compared with the IDF MetS components. To better understand how adiponectin levels correlate with MetS signs, see the data in Table 1. The majority of the features examined showed that low adiponectin levels were statistically significant.
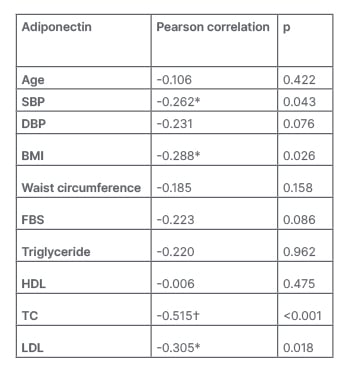
Table 1: Correlation between adiponectin levels and metabolic syndrome components.
*Correlation is significant at the 0.05 level (2-tailed).
?Correlation is significant at the 0.01 level (2-tailed).
DBP: diastolic blood pressure; FBS: fasting blood sugar; HDL: high-density lipoprotein; LDL: low-density lipoprotein SBP: systolic blood pressure; TC: total cholesterol.
As the number of MetS components rises, adiponectin levels decrease (p<0.001; Table 2).
According to multivariate analysis, the serum adiponectin concentration was significantly negative correlated with the SBP (r=-0.262; p<0.050), BMI (r=-0.288; p<0.050), TC (r=-0.515; p<0.001), and LDL (r=-0.305; p<0.050), respectively.
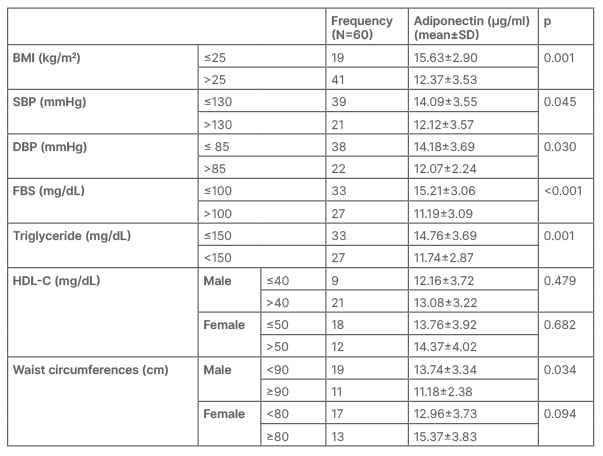
Table 2: Adiponectin levels decrease as metabolic syndrome components rise.
DBP: diastolic blood pressure; FBS: fasting blood sugar; HDL-C: high-density lipoprotein cholesterol; SBP: systolic blood pressure; SD: standard deviation.
In Figure 1, the receiver operating curve (ROC) shows that an adiponectin level with a cut-off <12.45 µg/mL revealed a sensitivity of 90.0%, specificity of 63.3%, and accuracy of 87.6% to predict the incidence of MetS in individuals.
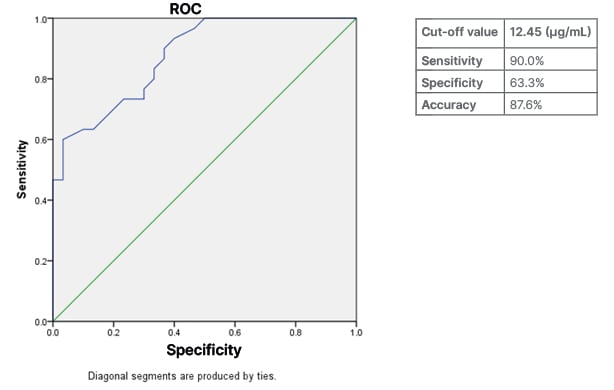
Figure 1: Predicting the prevalence of metabolic syndrome through the receiving operating curve.
ROC: receiving operating curve.
DISCUSSION
The current research was a 1-year cross-sectional study. It was used to investigate the relationship between adiponectin levels in MetS and other MetS components. Researchers Shashank et al.,19 Isa et al.,20 Chen et al.,21 Ntzouvani et al.,22 and Singh et al. 23 all used a similar methodology in their respective studies.
Isa et al.,20 Singh et al.,23 Chen et al.,21 and Shashank et al.19 utilised comparable approaches in their perspective studies. Results indicate that those without MetS were younger, had lower BMIs, and had smaller waist circumference measurements than those with MetS. They also had lower blood pressure, pulse rate, and fasting plasma glucose levels than the people with MetS, and there were statistically significant variations in lipid profiles between them and the patients with MetS, according to the findings. People without MetS had a greater mean serum adiponectin concentration (15.79±2.90 µg/mL) than those with MetS, who had a lower mean serum adiponectin concentration (11.02±2.63 µg/mL). The authors discovered that decreased adiponectin levels were statistically significantly linked with the majority of the characteristics of MetS. An increase in MetS components was observed to decrease adiponectin levels, which is statistically significant compared to previous studies.
SBP (r=-0.262; p<0.050), BMI (r=-0.288; p<0.050), TC (r=-0.515; p<0.001), and LDL cholesterol (r=-0.305; p<0.050) were all linked to blood adiponectin levels in this study. Patient adiponectin levels <12.45 ng/mL may be used as a cut-off to predict the occurrence of MetS in patients, according to the ROC, with a sensitivity of 90.0%, specificity of 63.3%, and an accuracy of 87.6%.
The mean baseline adiponectin level in participants with diabetes was lower than the mean baseline adiponectin level in subjects without diabetes (11.19±3.09 g/mL versus 15.21±3.06 mg/mL; p<0.001), according to this study. In a study performed by Chamukuttan et al.,24 adiponectin levels were shown to be lower in patients with diabetes (10 individuals) than participants without diabetes (23 individuals [11.3±5.5 versus 16.7±7.6 µg/mL; p=0.0017]). According to research by Singh et al.,23 adiponectin levels in patients with diabetes are lower than in participants without diabetes (6.07±1.02 versus 7.48±1.91 µg/mL; p=0.003). As reported by the study’s results, low levels of adiponectin in the blood are connected to T2D and to MetS.
Low adiponectin levels have been revealed to be a powerful predictor of future diabetes development, as well as a positive indication of that development. According to studies by Isa et al.,20 study participants who were hypoglycaemic exhibited no statistically significant variations in adiponectin levels compared to patients with normal glucose levels. The researchers hope to fill in the gaps by increasing the sample size and transitioning from a cross-sectional study to a cohort study with a longer follow-up period.
The adiponectin concentration observed in patients with hypertension (12.12±3.57 µg/mL) was lower than the concentration obtained in those without hypertension (14.09±3.55 µg/mL), and the link between the two was statistically significant. Furuhashi et al.25 reported that adiponectin concentrations were decreased in patients who are insulin-resistant and with essential hypertension, but not in patients who are normotensives or have hypertension but are non-insulin-resistant, indicating that hypoadiponectinemia in patients with essential hypertension is associated with insulin resistance. Both hypoadiponectinemia and insulin resistance are linked to the development of MetS, according to Renaldi et al.26
Males had adiponectin levels that were significantly higher than females (90 cm-13.74±3.34 µg/mL versus 90 cm-11.18±2.38 µg/mL; p=0.034), but females had lower levels (80 cm-12.96±3.73 g/mL versus 80 cm-15.37±3.83 µg/mL; p=0.094) and the association was insignificant. Males have more visceral fat and secrete more of the same hormones than females. Singh et al.23 found a significant association between waist circumference and circulating adiponectin, which was stronger than the connection between circulating adiponectin and BMI. This shows that visceral obesity (central fat distribution) rather than total fat mass is a better predictor of circulating adiponectin. P<0.001 was found for waist circumference in females more than 80 cm (5.98±1.18 mg/mL) compared with 9.90±2.70 mg/mL in females. In males greater than 90 cm (5.81±4.10 mg/mL) versus (7.90±0.05 mg/mL) was significant (p<0.001).
SBP (r=-0.262; p<0.050), BMI (r=-0.288; p<0.050), TC (r=-0.515; p<0.001), and LDL (r=-0.305; p<0.050) were all found to be inversely associated to serum adiponectin levels. According to Blaslov et al.,27 patients with higher adiponectin levels (n=39) had a significantly lower waist circumference (p=0.002), fasting venous glucose levels (p=0.001), higher HDL3-cholesterol (p=0.011), and estimated glomerular filtration rate (p=0.003) when compared to the group with lower adiponectin levels, which had a higher prevalence of MetS (p=0.045). Estimated glomerular filtration rate increased by 1.09mg/kg-1 min-1 for every 1 µg/mL rise in total fasting plasma adiponectin (p=0.003), with each additional gramme of adiponectin (p=0.003). In the logistic regression model, the presence of MetS was found to be inversely linked with the level of adiponectin (p=0.014). According to Taniguchi et al.,28 serum adiponectin levels were found to be negatively correlated with BMI (r=-0.308; p=0.002), diastolic blood pressure (r=-0.269; p=0.0012), and triglycerides (r=-0.338; p=0.001); and positively correlated with HDL-C (r=0.300; p=0.003). Similarly to this study’s findings, Chen et al.29 found that serum adiponectin was inversely linked with MetS.
Subjects without MetS had a higher mean serum adiponectin concentration (15.79±2.90 mg/mL) than those with MetS (11.02±2.63 mg/mL) with statistical significant difference as p<0.050 in this study. Adiponectin levels have previously been shown to vary by ethnicity, with African Americans having lower values.30,31 Asian Indians were shown to have lower adiponectin values than White people in a recent study by Abate et al.32 This study’s findings are noteworthy in this regard since they point to a strong correlation between adiponectin and MetS in Asian Indians. These data allow them to formulate a hypothesis that reduced adiponectin levels may be a contributing factor to the unexplained high level of insulin resistance seen among Asian Indians. This hypothesis might then be tested in prospective global research.
In the pre-set study, ROC showed that an adiponectin level with cut-off <12.450 µg/mL revealed a sensitivity of 90.0%, specificity of 63.3%, and accuracy of 87.6% to predict the incidence of MetS in individuals. In the prediction of MetS, adiponectin concentrations <7.425 µg/mL were shown to have high sensitivity (69.8%) and specificity (91.9%), as revealed by Singh et al.23 in their study. In the context of diabetes, plasma adiponectin levels may also be used to predict MetS; however, lower cut-off values are also recommended.
Plasma adiponectin may be a potential marker for MetS in the future, as each component of MetS was found to be strongly linked to the plasma concentration of adiponectin. The study’s most remarkable conclusion was that people in the lowest quartiles had a significantly greater risk of MetS than people in the highest quartiles when their mean adiponectin levels were compared. This study’s findings were supported by Singh et al.,23 Isa et al.,20 Taniguchi et al.,28 and Shashank et al.,19 in addition to other researchers.
The limitation of the study was the small size of the study group: 60 individuals. The participants in this study were middle-aged adults who did not have a history of CVD. Extrapolating the findings to other populations, such as those who are younger, of a different race, or who have CVD, should be done with caution.
The biochemical indices were evaluated by a single accredited laboratory throughout the whole process. When it came to evaluating the existence of MetS, the authors used IDF definitions. Currently, there are two definitions used to define MetS that are closely linked but differ mainly in the criteria for abdominal obesity. Finally, a study that gives a convincing explanation for the correlation between adiponectin and MetS has been released.
CONCLUSION
Current studies reveal that MetS lowers adiponectin levels in the circulatory system, and that these levels continue to fall as the number of MetS components in the population rises. The connection between adiponectin and HDL-C, triglycerides, fasting blood sugar, BMI, and waist circumference might explain why people with MetS have lower levels of adiponectin than the general population. To confirm the mechanisms that support this relationship, further prospective study is required.







