Abstract
Patients with diabetes who are infected with severe acute respiratory syndrome coronavirus 2 experience a worsening of glycaemic control and are at increased risk for severe outcomes. Little is known regarding the impact of COVID-19 vaccinations on glycaemic control. This case report explores a patient with diabetes who experienced an acute worsening of glucose control in the week following the second dose of the Pfizer-BioNTech COVID-19 vaccine.
Key Points
1.Patients with diabetes are at risk of experiencing a worsening of glycaemic control and severe outcomes if they are infected with severe acute respiratory syndrome coronavirus 2; however, little is known regarding the impact of COVID-19 vaccination.2. This case report presents a 66-year-old male who experienced an acute worsening of glucose control in the week following the second dose of COVID-19 vaccine.
3. More research is needed to clarify the pathophysiologic mechanism of hyperglycaemia caused by the COVID-19 vaccine, as patients may require closer follow-up or self-monitoring following vaccination.
INTRODUCTION
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide since December 2019.1 Though SARS-CoV-2 is spread primarily via respiratory droplets, the virus can produce a wide range of systemic signs and symptoms.2-4 In addition to adverse effects on the lungs, kidneys, nervous system, and cardiovascular system, COVID-19 affects the endocrine system, including causing hyperglycaemia in patients with and without diabetes.5 Several mechanisms have been proposed by which COVID-19 leads to hyperglycaemia. First, a large burden of inflammatory cells and markers such as IL-6, ferritin, D-dimer, erythrocyte sedimentation rate, and C-reactive protein affect the functions of skeletal muscle and the liver, the major insulin-responsive organs that are responsible for the bulk of insulin-mediated glucose uptake.6-8 Furthermore, as shown by Yang et al.,9 the SARS-CoV-2 virus can enter and destroy the islet cells of the pancreas via the angiotensin-converting enzyme 2 (ACE2) receptor, leading to decreased insulin production. Additional research by Hoffman et al.10 proved that the SARS-CoV-2 virus also uses the same ACE2 receptor.
As of 16th January 2022, over 63 million people in the USA have been infected by the SARS-CoV-2 virus and over 838,000 have died because of the COVID-19 disease.11,12 In December 2020, vaccines produced by Pfizer (Mainz, Germany) and Moderna (Cambridge, Massachusetts, USA) against SARS-CoV-2 received emergency use authorisation by the U.S. Food and Drug Administration (FDA) for the prevention of COVID-19.13 According to data from the Phase III clinical trials that led to approval, the most common side effects of the vaccines include fever, fatigue, headache, chills, and injection site pain. Neither Phase III trial reported hyperglycaemia as an adverse event.14,15 The Vaccine Adverse Events Recording System (VAERS), a collection of unconfirmed vaccine adverse events submitted by healthcare providers, manufacturers, and the public, reports 438 cases of hyperglycaemia following vaccines. According to VAERS, there have been 105 cases of reported hyperglycaemia following COVID-19 vaccination with any brand of vaccine, 58 of which occurred following the Pfizer-BioNTech vaccine and 46 after the Moderna vaccine.16
The authors conducted a literature search regarding the impact of vaccines, particularly for COVID-19, on glucose control in patients with diabetes. The only report in the literature regarding post-vaccine hyperglycaemia was a patient with well-controlled diabetes who experienced self-limited hyperglycaemia for 72 hours following the influenza vaccine.17 There is no published literature regarding hyperglycaemia in latent autoimmune diabetes of adulthood or Type 1 diabetes following the COVID-19 vaccine. In this case, the authors describe a patient with diabetes who experienced worsened glycaemic control in the first 7 days after they received their second dose of the Pfizer-BioNTech COVID-19 vaccine in February 2021.
CASE DESCRIPTION
This case is a 66-year-old Indian-American male with latent autoimmune diabetes of adulthood in the setting of diabetic ketoacidosis. They have no history of macrovascular or microvascular complications of diabetes, and their most recent glycated haemoglobin tests were 6.3% (45 mmol/mol) on 9th November 2020, and 7.0% (53 mmol/mol) on 4th March 2021. Prior to receiving the Pfizer-BioNTech COVID-19 vaccine in February 2021, the patient was using insulin degludec 20 units at night; insulin aspart with an insulin to carbohydrate ratio of 1 unit: 8 g with breakfast and 1 unit: 10 g with lunch and dinner; insulin aspart with a correction of 1 unit for every 40 mg/dL over 140 mg/dL; and metformin 500 mg twice daily. These medications and doses had not changed in over 3 months. The patient monitors their glucose with a Dexcom (San Diego, California, USA) continuous glucose monitor.
The patient received the first dose of the vaccine on 6th February 2021. In the two weeks leading up to this (23rd January–6th February: Time A), their average daily glucose was 188.7 mg/dL, and their average total insulin use was 44.1 units. They received the second dose of the vaccine on the 26th February. In the time between the two doses (7th February–26th February: Time B), their average daily glucose was 184.3 mg/dL and their average total insulin use was 44.1 units. In the 7 days following the second dose (27th February–5th March: Time C), their average daily glucose increased to 198.8 mg/dL, despite an increase in the average total insulin use of 56.7 units. From 5th March–19th March (Time D), their average daily glucose decreased to 160.6 mg/dL and average total insulin use to 44.5 units (Figure 1). According to the patient’s continuous glucose monitor, they were in target glucose range 49% of the time during Time A, 61% of the time during Time B, 50% of the time during Time C, and 70% of the time during Time D.
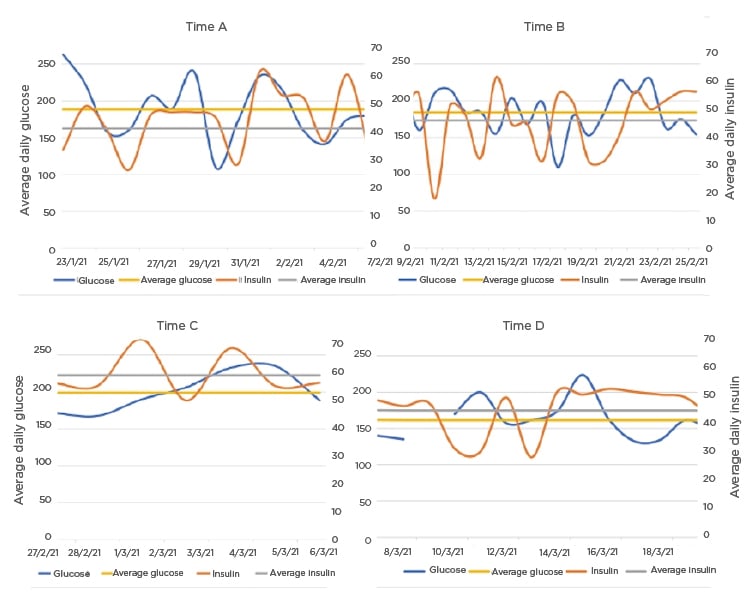
Figure 1: The patient’s average daily glucose levels and total daily insulin use across Times A, B, C, and D, as well as average glucose and average daily insulin during each time period.
RESULTS
Student’s T-tests were used to compare the patient’s total daily insulin use among the four time periods (A–D). There was no difference in total daily insulin use between Time A and B (p=0.992). However, the authors found a significant difference Time B and C (p=0.004) and between Time C and D (p=0.005). The patient’s average daily glucose was also analysed: there were no significant differences found between Time A and B and between Time B and C (p=0.737 and p=0.262, respectively), but there were significant differences between Time C and D (p=0.019). The patient’s daily carbohydrate intake was also analysed, and there was only a significant increase found between Time C and D (p=0.006 [ Table 1 ]).
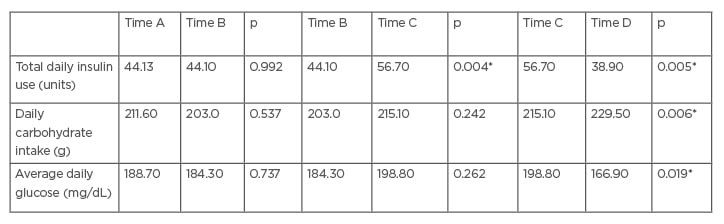
Table 1: The total daily insulin use, carbohydrate intake, average daily glucose, and homeostatic model assessment of insulin resistance for the time periods of interest.
Significant differences were found in total daily insulin usage between Time B and Time C, as well as when comparing Time C with Time D. Average daily glucose between Times C and D were also significant. There was also a significant increase in daily carbohydrate intake between Time C and D (p=<0.05*).
DISCUSSION
The authors report a case of a patient with well-controlled latent autoimmune diabetes of adulthood that experienced an acute worsening of glucose control in the week following the second dose of the Pfizer-BioNTech COVID-19 vaccine. They analysed the average total daily insulin used by the patient and average glucose levels before receiving the first vaccine dose, in between the two vaccines doses, the week following the second vaccine dose, and after the second dose. The statistics show that glucose levels peaked in the week following the second dose of the vaccine compared to the time immediately before and immediately after receipt of the vaccine, despite a statistically significant increase in insulin use. Though the average daily glucose was statistically higher only when comparing the levels during the first week following the second dose (Time C) with the levels beyond that time (Time D), the levels immediately following the second dose showed a clear trend towards statistical significance when compared with earlier levels (Times A and B). However, during this time, the patient was using significantly increased levels of insulin to keep their glucose levels in a similar range. This further supports the use of average daily insulin as a surrogate for glucose control. There were no other changes reported by the patient that might explain the acute worsening of glycaemic control, including dietary changes, infection, stress, or medication use. The patient’s daily carbohydrate intake during these time periods showed no significant changes. This helps eliminate any doubt that the increase in total daily insulin requirement following the vaccine was due to changes in dietary habits.
A possible cause of the patient’s acute worsening of glycaemic control could be due to the fact that the Pfizer-BioNTech is an mRNA vaccine. Compared with DNA vaccines, mRNA vaccines work through inflammatory pathways such as toll-like receptors 3, 7, and 8, leading to greater systemic inflammation.18 It is possible that the inflammatory effects of the vaccine impacted this patient’s glucose regulation. Given that the patient has latent autoimmune diabetes of adulthood, the authors can presume that it is an increase in insulin resistance, rather than a decrease in beta cell function and insulin production, that caused the increase in insulin requirements.
A weakness of this study is that the carbohydrate intake described was inputted into the continuous glucose monitor and, therefore, self-reported by the patient. This leaves room for the possibility of error in reporting that could have an impact on the results. However, the patient has demonstrated extensive knowledge in the past about their disease; all meals are cooked and eaten at home; and they have a history of properly taking their medications and documenting their carbohydrate intake. Another weakness is that the authors cannot be completely certain that the patient’s acute hyperglycaemia was due to the vaccine. The patient was in the hospital from the 4th January to 11th January 2021 for post-infection vertigo, following a left ear infection. It is possible, but very unlikely, that the stress from this hospitalisation had an impact on the patient’s glucose control. Before their hospital stay, the patient was treated with levofloxacin, an antibiotic that has been shown to cause issues with glucose control.19 However, this is less likely because statistically significant change in their glucose control occurred over 1 month after this hospitalisation and use of the medication.
While acute hyperglycaemia is known to occur following infection with the SARS-CoV-2 virus, it is not clear if this is due to pancreatic β-cell dysfunction or increased insulin resistance. The COVID-19 virus interacts with the ACE2 receptor, potentially causing pancreatic islet cell destruction, leading to decreased insulin release and hyperglycaemia. Given that the mRNA vaccines encode the SARS-CoV-2 full-length spike rather than an attenuated version of the virus, the authors would not expect that the mechanism hyperglycaemia following the Pfizer-BioNTech COVID-19 vaccine is the same for the virus and the mRNA vaccine.9,10 However, there is no currently available literature about how and if the vaccine’s SARS-CoV-2 full-length spike interacts with the ACE2 receptor. This case, as well as the unconfirmed cases reported by VAERS, illustrate that hyperglycaemia is an adverse event that can be seen following COVID-19 vaccination. This finding is important because hyperglycaemia can lead to the life-threatening complications of diabetic ketoacidosis and hyperosmolar hyperosmotic state in patients with diabetes. As a result of poorer clinical outcomes following infection with the SARS-CoV-2 virus, patients with diabetes are considered to be at a high-risk for severe disease.20,21 Studies show that patients with new-onset or pre-existing diabetes who are infected with COVID-19 are more likely to be admitted to the intensive care unit and have increased disease severity and mortality, making primary prevention of infection in this population essential.22,23 Subsequently, there is a greater push for this population to receive a COVID-19 vaccination.20-23 In fact, as of 2nd April 2022, there were over 114 million people in the USA who were not yet completely vaccinated.24,25 With an estimated 10.5% of the USA’s population having diabetes, and 34.5% having pre-diabetes, this meant that there were 120.4 million Americans who may have been at risk of poor glycaemic control following COVID-19 vaccination.26
With the knowledge that hyperglycaemia can occur following the Pfizer-BioNTech COVID-19 vaccine, clinicians should have close follow-up with patients with diabetes and monitor glucose and insulin levels. Additionally, patients with diabetes and their physicians should be educated about this possible side effect. There must be awareness amongst this population so the proper steps can be taken if a patient experiences hyperglycaemia or a worse sequela following vaccination.
Ultimately, there is still much to learn about the relationship between worsening glucose control and the COVID-19 vaccine. Continued reporting of this side effect will help with this effort so the patient population can be better understood. Additional studies to elucidate the mechanism by which the Pfizer-BioNTech effects glucose metabolism will also be useful. Greater understanding of how this vaccine can induce worsening of hyperglycaemia may help us further understand how COVID-19 induces hyperglycaemia, why hyperglycaemia is associated with poorer outcomes from COVID-19, and how this and future mRNA vaccines can be used safely.
SUMMARY
While VAERS reports cases of hyperglycaemia following COVID-19 vaccination, this is among the first cases reported in scientific literature. Fortunately, the authors’ patient closely monitors their glucose levels and adjusts their insulin appropriately. Other patients with diabetes who are not as attentive may have poor outcomes because of Pfizer-BioNTech COVID-19 vaccine-induced hyperglycaemia. Given the novelty of the COVID-19 virus and the mRNA vaccines, it is essential that these cases are reported to add to the growing body of literature. More research must be undertaken to elucidate the pathophysiologic mechanism of hyperglycaemia caused by the Pfizer-BioNTech COVID-19 vaccine. Cases such as this will also show which patients may require closer follow-up or self-monitoring following vaccination. This also allows patients and physicians to better understand the risks and possible side effects of vaccination given specific comorbidities.