Abstract
Moringa oleifera leaf powder (MOLP) was incorporated into patient diets in order to study its effects on the levels of fasting blood glucose (FBG), glycated haemoglobin (HbA1c), serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and body weight (BW) in those at the early stages of Type 2 diabetes mellitus (T2DM). Two tablespoons (20 g) of the leaf powder were added to a basic diet of food served cold daily at lunch and dinner for a period of 3 months. Pre-diabetic control subjects were given the basic diet without MOLP. The supplementation of MOLP into the basic diet significantly (p<0.05) reduced the elevated FBG, HbA1c, TG, TC, and LDL cholesterol levels in the MOLP-diet group, while an increase in HDL cholesterol was also recorded. MOLP exerted more pronounced effects at the end of the study when compared with the control group. Overall, BW was reduced, with better results recorded in the MOLP group. Considering the changes when compared to each initial value, the efficacy of the MOLP diet on biochemical parameters was 3.55–24.79% greater. The introduction of the effective potential change revealed an efficacy induction of 8.85–36.83% due to the MOLP diet, with a relative performance factor ranging from 1.50–4.85 among the biochemical parameters. The findings suggest that MOLP possesses promising anti-hyperglycaemic, anti-hyperlipidaemic, and lipid profile regulatory properties in T2DM subjects.
INTRODUCTION
Diabetes mellitus has been defined as a disease characterised by chronic elevated blood glucose concentration, as a result of deficiency or the diminished effectiveness of insulin. The use of local plant materials for the treatment of infectious and non-infectious diseases is widely developed in the traditional medicine of tropical countries. Such plants have enormous therapeutic potential and have been traditionally used to treat various diseases (e.g. malaria, asthma, dysentery, ulcers, diarrhoea, and Type 2 diabetes mellitus [T2DM]). The correct and intelligent exploitation of knowledge regarding the disease healing properties of medicinal plants could help to considerably improve the health of the population and, thus, contribute indirectly to sustainable economic growth and poverty reduction in many countries of the tropics.
Diabetes mellitus, particularly T2DM, and coronary heart disease are considered ‘civilisation diseases’, which are spreading among the populations in the tropics because of changes in diet and lifestyle. It is known that the treatment of these diseases in modern medicine is very expensive and many anti-diabetic drugs seem to pose a significant risk for the patient. The management of diabetes with fewer or no side effects is still a challenge to medical systems. The use of herbal medicines for the prevention or treatment of T2DM has thus gained importance throughout the world. Various studies have been conducted on a selection of plant extracts, which have had promising results for the prevention band treatment of chronic non-infectious diseases. These plants are said to contain substances that possess anti-hyperglycaemic, anti-hyperlipidaemic, and anti-hyperproteinaemic properties.1
Moringa oleifera leaf is widely used by populations in the tropics as a vegetable in the kitchen as well as a food supplement. The plant commonly referred to as the ‘miracle tree’ has been found to be useful in the ethnotherapy of undernutrition and possesses anti-cancer, anti-inflammatory, and hepatoprotective effects.2-4 The anti-diabetic potential of the M. oleifera leaf extract has previously been reported.5-7 The present study was undertaken to investigate the anti-hyperglycaemic and anti-hyperlipidaemic effects of M. oleifera leaf powder (MOLP) in patients at an early stage of T2DM.
MATERIALS AND METHODS
M. oleifera leaves were collected in a field in Akassato village, 8 km from Cotonou, the economic capital city of Benin, which is bordered by the Atlantic Ocean. The plant material was washed completely (2–3 mins) with tap water to remove contaminants and rinsed briefly with distilled water. The washed leaves were air and shade dried at room temperature (27oC±2) for 4 weeks and made into powder using pestle and mortar. The powder was sieved into a finer powder using a traditional sieve, where coarse samples (negligible mass of petiole) were removed. The fine leaf material was stored in a traditional wooden container at room temperature prior to use.
Characteristics of Participants
A total of 20 free-living diabetic males at the early stages of T2DM (i) and 20 pre-diabetic patients as the control (ii) were selected, with the following prerequisites: i) fasting blood glucose (FBG): 130.7±2.2 mg/dL; age: 51±4 years; body weight (BW): 87.2±6.3 kg; BMI: 31.2±3.4; ii) FBG: 114.20±3.0 mg/dL; age 48±2; BW: 84.7±4.5 kg; BMI: 29.26±2.1.
Hyperglycaemia had been diagnosed in the subjects within the 4 months prior to commencing the study. No relevant history of coronary heart disease, renal, hepatic, respiratory, or other endocrine dysfunction had been observed in the patients. A written consent form was signed by the participants to ensure consistency with the ethical standards in place when human patients are used in a research study.
Diet
The patients’ diet was composed of local African foods: cooked maize or sorghum mill served with okra or Amaranthus hybridus sauce prepared with tomato, fish, onion, and soja (soybean) oil. The fat and protein in this diet are mostly from vegetable sources and fish. Water prepared tubers were allowed once a week. Upon consumption, the diabetic patients received two tablespoons (20 g) of MOLP mixed with the cold served food of the basic diet for lunch and the same quantity (20 g) for dinner. The pre-diabetic control patients received only the basic food. For breakfast a small portion of bread and vegetables, such as cucumber, was permitted.
Recommendations
Most of the participants were aware of MOLP and 90% of the diabetic subjects had consumed the powder with other medicinal plants prior to the study, albeit uncontrolled and irregularly. It was advised that all participants should avoid the following foods: oily foods, oil rich in saturated fatty acids, sweet foods, imported industrial foods, alcohol or alcoholic drinks, sweet drinks, and tobacco or tobacco products. Only small portions of local fruits and vegetables including mango, banana, and cucumber were additionally permitted (after lunch and dinner). Each subject was instructed to avoid sedentary habits and to practice physical activity with a minimum of 30 minutes walking time required per day particularly after dinner intake. Dinner intake was fixed between 6 pm and 7 pm. All subjects were pre-treated with anti-malarial drugs before starting the study and long lasting impregnated mosquito nets had been distributed to them with the recommendation to sleep under the nets. This is a preventive measure to avoid malaria infection and illness which can influence the blood parameters, particularly the value of the glycated haemoglobin (HbA1c) in the case of anaemia.
Biochemical Parameters
At each evaluation time, venous blood was drawn from both pre-diabetic control patients and the T2DM patients after 12 hours overnight fasting, and serum was separated. Blood glucose concentrations were estimated using glucose oxidase-peroxidase reactive strips and a glucometer (Accu-Chek®, Roche, Basel, Switzerland). HbA1c was measured by immunoassay (IMx, Abbott, Illinois, USA); total cholesterol (TC), high-density lipoprotein (HDL) cholesterol were assayed by routine laboratory methods. The low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald equation.8
Effective Potential Change
As the initial values of the subject groups were different, a mathematical formula was developed to calculate the effective potential change (EPC) value in the MOLP group when compared to the control for all tested parameters at the end of the study period:
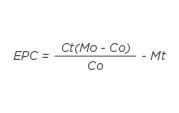
Where Ct is the control diet value at time (t) after the start of the study, Mo is the initial plant material diet value, Co is the initial control diet value, and M is the plant material diet value at time (t) after the start of the study.
Statistics
Data were presented as the mean±standard error of the mean and was analysed using one-way analysis of variance using the IBM® SPSS® Modeler 16.0 computer software package (IBM, New York, USA). Differences at p<0.05 were considered significant.
RESULTS
Fasting Glucose and Glycated Haemoglobin
In the MOLP group, HbA1c and FBG decreased continually. Significant effects (p<0.05) occurred in HbA1c level after 1 month and were more pronounced after 2 and 3 months. Significant reductions in FBG concentration were observed after 2 and 3 months in comparison with the initial value (Table 1). The two parameters decreased progressively in the control-diet group with pronounced diminutions (p<0.05) after 3 months. In spite of the differences between the initial values, the recorded data in the MOLP group were closer to the corresponding values in the control group at the end of the trial (FBG: 105.4 mg/dL versus 102.1 mg/dL; HbA1c: 5.28% versus 5.0%).
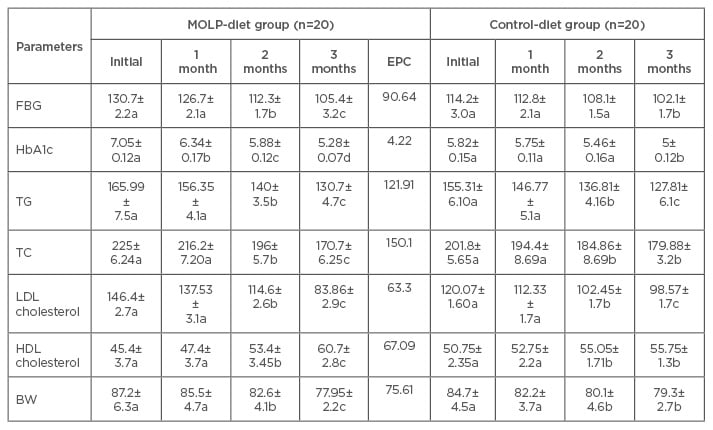
Table 1: Changes in the levels of fasting blood glucose (mg/dL), glycated haemoglobin (%), lipid profile (mg/dL), and body weight on treatment with Moringa oleifera leaf powder.
Values are mean±standard error of the mean.
Values of each parameter, followed by a different letter, which differ within the same line, are significantly different with respect to the previous value at p<0.05.
FBG: fasting blood glucose; HbA1c: glycated haemoglobin; TG: triglyceride; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein; BW: body weight; MOLP: Moringa oleifera leaf powder; EPC: effective potential change.
Blood Lipid Profile
After consumption of MOLP, the levels of serum triglyceride (TG), TC, and LDL cholesterol decreased continually with significant (p<0.05) reductions after 2 and 3 months. The reduction at 3 months was the highest. In the control group, progressive significant decreases (p<0.05) were observed after 2 and 3 months with more pronounced effects on TG and LDL cholesterol. The HDL cholesterol concentrations increased in both control-diet and MOLP-diet groups during the entire trial period with significant (p<0.05) effects after 2 and 3 months, but the increase was statistically greater in the MOLP-diet group at the end of the study.
Body Weight
The results indicate that the dietary restrictions induced progressive diminutions in both the control and MOLP-diet groups. After 3 months, the BW reduction was significantly greater (p<0.05) in the MOLP-diet group (9.25 kg) than that observed in the control-diet group (5.4 kg).
Effective Potential Changes
The percentage changes in the biochemical parameters at the end of the trial period are presented in Table 2 and illustrated in Figure 1. FBG, HbA1c, TG, TC, and LDL cholesterol were reduced overall, while increases in HDL cholesterol have been observed in both the control and MOLP-diet groups. Considering the changes when compared to each initial value, the efficacy induced by the MOLP diet was 3.55–24.79%, which was greater than those observed in the control group.
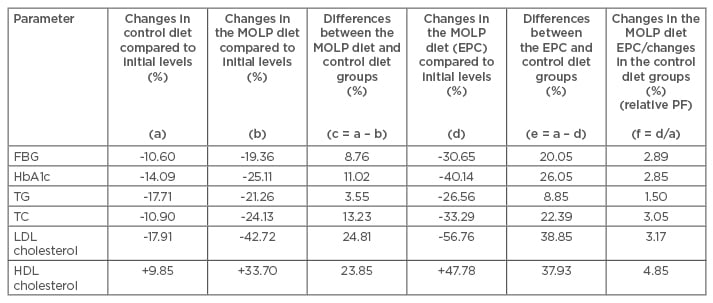
Table 2: Percentage changes in the biochemical parameters of the control and Moringa oleifera leaf powder groups at 3 months based on the effective potential changes under the M. oleifera leaf powder diet.
MOLP: Moringa oleifera leaf powder; EPC: effective potential change; PF: performance factor; FBG: fasting blood glucose; HbA1c: glycated haemoglobin; TG: triglyceride; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
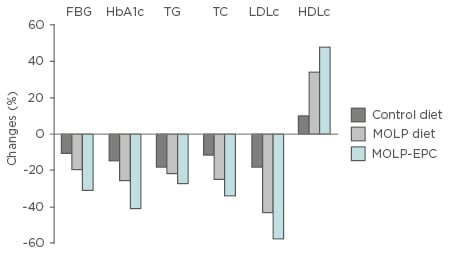
Figure 1: Changes in biochemical parameter concentrations after 3 months of consumption of Moringa oleifera leaf powder in diabetic and control subjects.
Control-diet: percentage change from basic diet to initial control value; MOLP-diet: percentage change from basic diet + MOLP to initial MOLP group value; MOLP-EPC: effective potential change of MOLP diet to initial MOLP group value.
MOLP: Moringa oleifera leaf powder; EPC: effective potential change; HbA1c: glycated haemoglobin; FBG: fasting blood glucose; TG: triglyceride; TC: total cholesterol; LDLc: low-density lipoprotein cholesterol; HDLc: high-density lipoprotein cholesterol.
Based on the calculated EPC value after 3 months, the MOLP diet induced 8.85–38.83% more effect among the biochemical parameters when compared to the control diet, with the highest effect on the LDL cholesterol and HDL cholesterol levels. The effect of the combination MOLP diet calculated as a relative performance factor was 1.50 to 4.85-times greater than those observed in the control diet alone among the biochemical parameters.
DISCUSSION
A radical change in diet and lifestyle is a safe strategy for T2DM patients. Hyperglycaemia is often associated with an increase in blood lipid levels and can lead to, in the worst case, fatal complications. The short diet restriction period of 3 months showed that the consumption of MOLP as a supplement is a means of maintaining blood glucose and lipid concentrations in the normal medically defined ranges. The uncontrolled consumption of foods over many years (particularly foods rich in fat and carbohydrates) leads to obesity, T2DM, and other chronic non-infectious diseases such as cardiac disease or liver cirrhosis. All subjects enrolled in this study were overweight or obese and had a history of consuming fatty foods in combination with a sedentary lifestyle.
In the present study, the mean BW and BMI reductions were more pronounced in subjects supplemented with MOPL (BMI: 31.20–27.94) in comparison with the control-diet group (BMI: 29.26–28.22). The majority in the MOLP-diet group reported a reduction in their daily eating frequency following MOLP diet consumption, which is probably one of the causes of the weight management property of the plant material. Hyperglycaemia in T2DM is characterised by insulin resistance, which is responsible for the transport of glucose from the blood to the cells. FBG and HbA1c serve to measure the glycaemic status of the patient. HbA1c indicates the average plasma glucose over the previous 8–12 weeks. In contrast to FBG, it can be tested at any time without any preparation in the case of absence of infection, which can affect the red blood corpus. In the present study, FBG, HbA1c, and blood lipid concentrations were measured. In the initial blood lipid values of the subjects, only the TG level exceeded its upper defined limit of 150 mg/dL (1.70 mmol/L). This is probably due to the fact that the most popular African foods are prepared with oils rich in saturated fatty acids which are cheaper than oils rich in unsaturated fatty acids, such as peanut or soy oil. The permanent consumption of vegetable oil rich in saturated fatty acids can exert negative effects on the endogenous lipid profile.
After the basic dietary incorporation, the levels of all tested parameters progressively improved in the control group and remained significant (p<0.05) at the end of the study period. The initial elevated concentration of these parameters was significantly reversed in diabetic patients (p<0.05), particularly from Month 2 until the end of the trial period after consumption of MOLP as a supplement. FBG and TG concentrations in diabetic patients returned to within the defined upper limits of 110 mg/dL (6.1 mmol/L) and 150 mg/dL (1.70 mmol/L), respectively, at the end of the study period.
In spite of the differences between the initial values (in benefit of the control), the glucose-lowering and the positive blood lipid regulatory properties of the MOLP diet were greater than the control diet. This was illustrated by the introduction of the new mathematical formula for EPC. The EPC formula was developed for proper elucidation of the optimal performance of the MOLP diet. The MOLP-diet group observed better performance on the levels of the tested biochemical parameters when compared to the control group, demonstrating that MOLP possesses anti-hyperglycaemic and blood lipid profile regulatory properties. Medicinal plant extracts had earlier been reported to have anti-hyperglycaemic and lipid-lowering properties.9-13 M. oleifera leaf extract has been found to reduce hyperglycaemia, dyslipidaemia, and T2DM.14,15
The prompt initiation of treatment measures to manage the blood glucose level in diabetic patients is a key strategy to counter or prevent the multiple dysfunctions of lipoprotein metabolism and other angiopathies in diabetes, which are high health-risk factors for the individuals concerned. It should be noted that lipid metabolism in the blood system is very complex. The cholesterol-binding lipoproteins are playing different roles inside the blood vessel. LDL transports cholesterol to the cells and can cause atherosclerosis in the case of depot failure in cells, due to, for example, a dysfunction of the LDL receptors. HDL promotes the reverse cholesterol transport route in avoiding excess cholesterol accumulation and in preventing the generation of an oxidised and modified LDL,16 or by removing cholesterol from the arteries and transporting it back to the liver where it can be eliminated from or used again by the body. Insulin resistance or deficiency can lead to hyperglycaemia and can oblige the adipose tissues to release fatty acids for energy needs. These acids then accumulate in the liver and are converted into TG.17 Increased TG, LDL, and TC creates a high risk of atherosclerosis in diabetic patients. The highly significant reduction of these parameters suggests that the M. oleifera leaf possesses substances which can prevent atherosclerosis and heart infarcts in diabetic patients. The plant extract has been reported to exert cholesterol-lowering4,18 and cardio-protective19 effects in humans as well as in experimental animal models.
In the present study, the modes of action of the phytocomponents in the plant material, which are responsible for the observed effects in the diabetic subjects, have not been investigated. It should be noted that phytochemicals such as bioflavonoids in the M. oleifera leaf can play a key role in glucose stimulation and regulation, and carbohydrate metabolism.20 The lipid-lowering activities of the M. oleifera leaf extract have been attributed to the bioactive phytoconstituent sitosterol.21 Antioxidant and hepatoprotective potentials of the M. oleifera leaf extract may be due to the presence of total phenolics and flavonoids, plus active constituents like b-sitosterol, quercetin, and kaempferol.22 The atherosclerotic preventive role of MOLP through inhibition of LDL cholesterol oxidation was attributed to a bioactive phytocomponent called β-carotene.11 T2DM is associated with oxidative stress leading to derangements in the metabolism of proteins, lipids, and carbohydrates. In the present study, the consumption of MOLP exerted the highest lipid-lowering effect on LDL cholesterol. Antioxidant activities of M. oleifera and other medicinal plants and their roles in disease therapy have been reported in various studies.5,23-27 The observed induced blood glucose-lowering and lipid regulatory effects of MOLP could be due to the presence of the phytoconstituents and antioxidants in the M. oleifera leaf, which was probably ameliorated in treated subjects by key biomarkers (adiponectin, resistin, and leptin) involved in obesity, lipid function, and insulin sensitivity as has been demonstrated in experimental models.28,29 Further investigations should be conducted to elucidate the effects of the responsible plant bioactive substances on the biochemical mechanisms involved in the observed results.
CONCLUSION
The observed results in this study indicate that both a radical dietary restriction and dietary supplementation of MOLP have positive blood sugar and blood lipid profile regulatory effects by increasing HDL, which is beneficial, and reducing FBG, HbA1c, TG, TC, and LDL, which are high-risk health factors for diabetic patients.