Abstract
The micro and macrovascular complications of Type 2 diabetes mellitus are influenced by several well described cardiovascular risk factors such as hyperglycaemia, hypertension, hyperlipidaemia, and smoking alongside age, sex, and diabetes duration. Modern guidelines have defined treatment and goals for these risk factors based on evidence. As new trials are constantly published, these risk factors must be analysed for evidence to contribute to guidelines that are being revised. During recent years three new trials (EMPA-REG, LEADER, and SUSTAIN-6) have shown that treatment of hyperglycaemia with new anti-diabetic drugs has been able to reduce a composite cardiovascular endpoint. This is a great achievement and is the focus of this review, which also summarises developments in the treatment of other relevant risk factors. Ultimately, a high-quality level of diabetes care also needs to involve a well-informed and motivated patient; if compliance is suboptimal the benefits of modern treatment will not be reached.
INTRODUCTION
Type 2 diabetes mellitus is associated with increased cardiovascular risk as documented in numerous observational studies. This is caused by the impact of cardiovascular risk factors such as hypertension, hyperlipidaemia, hyperglycaemia, and increased risk of thrombosis besides the influence of age, sex, and diabetes duration. The mechanisms linking these risk factors with disease manifestation of micro and macrovascular complications are based on gene-environmental interactions, where a less healthy lifestyle, for example smoking, lack of physical exercise, or unhealthy eating habits, result in obesity.
Several international guidelines have addressed the challenge of screening, diagnosing, and treating these risk factors, not only in patients with Type 2 diabetes mellitus but also in subjects with impaired fasting glucose or impaired glucose tolerance.1 In the USA, the American Diabetes Association (ADA) published the annual ‘Standards of Medical Care’ in 2016, where updated recommendations for the treatment of Type 2 diabetes mellitus and risk factor control were stated.2 Within these guidelines goals have been set for the control of glycaemia, blood pressure, lipids, and smoking cessation.1,2 A summary of current goals set for risk factor control in Europe along with some comments are listed in Table 1.1
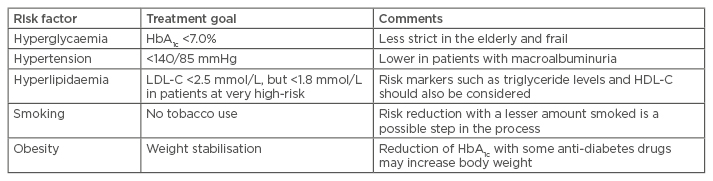
Table 1: Treatment goals for risk factor control in patients with Type 2 diabetes mellitus.1
LCL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
During 2013–2016 several important new clinical intervention trials in patients with Type 2 diabetes mellitus have been published to expand the evidence base for how to deal with the risk factors regarding drug therapy. This is not a way to diminish the important role of lifestyle modifications but the evidence from drug interventions should now be updated and critically discussed. In this brief review some of the newer studies have been reviewed for their main findings and commented upon, with a focus on newer anti-diabetic drugs.
TREATMENT OF HYPERGLYCAEMIA
The new drug alternatives to treat Type 2 diabetes mellitus have been tested in several recent large- scale intervention trials in recent years. Of these trials, three have included a dipeptidyl peptidase 4 (DPP-4) inhibitor versus placebo, three a glucagon- like peptide-1 (GLP-1) receptor agonist/analogue (GLP-1 RA) versus placebo, and one a sodium/ glucose cotransporter 2 (SGLT2) inhibitor versus placebo. The patients with diabetes recruited have generally been high-riskpatients and in most cases, with a previous cardiovascular disease (CVD) manifestation. This is both a merit and a problem, as it is not self-evident that findings in these high-risk patients can be extrapolated to other patients with diabetes but at a lower cardiovascular risk (Table 2).
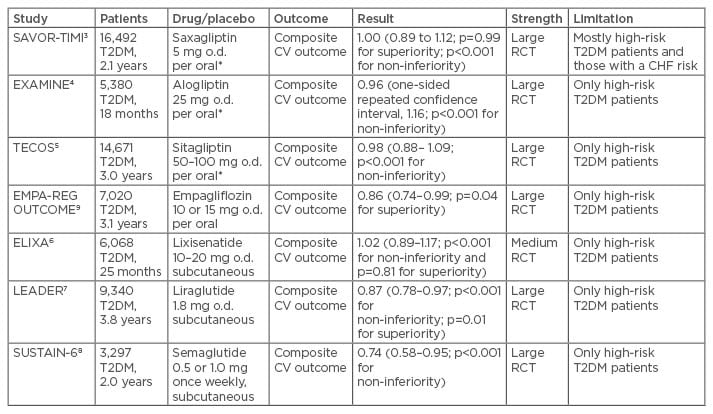
Table 2: Summary of outcome trials with new anti-diabetic drugs.
Risk ratios with 95% confidence intervals and significance testing for non-inferiority and superiority.
*Reduced dosage in patients with impaired renal function estimated glomerular filtration rate (eGFR) 30–60 mL/min.
T2DM: Type 2 diabetes mellitus; CHF: congestive heart failure; RCT: randomised controlled trial; CV: cardiovascular; o.d.: once daily.
The three DPP-4 inhibitor trials were SAVOR-TIMI 53,3 EXAMINE,4 and TECOS,5 with an overall favourable safety profile, the only exception being the increased risk of congestive heart failure as noted in the SAVOR-TIMI 53 trial.3 In all three trials, non-inferiority against placebo was shown but the cardiovascular event risk was not different from placebo. Thus, these drugs are generally safe but did not provide added benefits for cardiovascular prevention. The next set of trials were the three GLP-1 RA studies ELIXA,6 LEADER,7 and most recently the SUSTAIN-6 study,8 from which the latter two will be related in more detail due to their positive outcomes. On the other hand, the ELIXA study showed non-inferiority and safety but no added cardiovascular prevention versus placebo in patients with Type 2 diabetes mellitus and a recent event of an acute coronary syndrome as inclusion criterion.6 Finally, the EMPA-REG OUTCOME trial tested the new class of SGLT2 inhibitors for non-inferiority and cardiovascular effects versus placebo.9 This trial will also be described more in detail.
LEADER
In the double-blind LEADER trial, patients with Type 2 diabetes mellitus and high cardiovascular risk were randomised to receive liraglutide or placebo.7 The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke. The primary hypothesis was that liraglutide would be non-inferior to placebo regarding the primary outcome, with a margin of 1.30 for the upper boundary of a 95% confidence interval (CI) of the hazard ratio (HR). A total of 9,340 patients underwent randomisation with a median follow-up of 3.8 years. The primary outcome occurred in significantly fewer patients in the liraglutide group (608 of 4,668 patients [13.0%]) than in the placebo group (694 of 4,672 [14.9%]) (HR: 0.87; 95% CI: 0.78–0.97; p<0.001 for non-inferiority; p=0.01 for superiority). Fewer patients died from cardiovascular causes in the liraglutide group (219 patients [4.7%]) than in the placebo group (278 [6.0%]) (HR: 0.78; 95% CI: 0.66–0.93; p=0.007). The rate of death from any cause was lower in the liraglutide group (381 patients [8.2%]) than in the placebo group (447 [9.6%]) (HR: 0.85; 95% CI: 0.74–0.97; p=0.02). The rates of non-fatal myocardial infarction, non-fatal stroke, and hospitalisation for heart failure were non-significantly lower in the liraglutide group. The most common adverse events leading to the discontinuation of liraglutide were gastrointestinal events. The authors concluded that the rate of the first occurrence of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke among patients with Type 2 diabetes mellitus at high-risk was lower with liraglutide than with placebo.7
The reduction of cardiovascular mortality is an important achievement taking into consideration that the patients in LEADER were mostly well treated already at baseline by several drugs for secondary prevention of CVD. For example, a very high proportion of the patients were treated with statins or renin-angiotensin system-blocking agents. An important contributing factor could be the weight loss induced by liraglutide (a mean difference of 2.3 kg at 36 months versus placebo [95% CI: 2.5-2.0])
SUSTAIN-6
The most recent GLP-1 RA trial was SUSTAIN-6, presented at the European Association for the Study of Diabetes (EASD) 52nd Annual Meeting in Munich, Germany, September 2016, and published simultaneously.8 The rationale was that the cardiovascular effects of semaglutide, a GLP-1 RA with an extended half-life of approximately 1 week, in Type 2 diabetes mellitus were unknown. A total of 3,297 patients with Type 2 diabetes mellitus on a standard-care regimen were randomly assigned to receive once-weekly semaglutide (0.5 mg or 1.0 mg) or placebo for 104 weeks. The primary composite outcome was the first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. It was hypothesised that semaglutide would be non-inferior to placebo for the primary outcome. The non-inferiority margin was 1.8 for the upper boundary of the 95% CI of the HR. At baseline, 2,735 of the patients (83.0%) had established CVD, chronic kidney disease, or both. The primary outcome occurred in 108 of 1,648 patients (6.6%) in the semaglutide group and in 146 of 1,649 patients (8.9%) in the placebo group (HR: 0.74; 95% CI: 0.58–0.95; p<0.001 for non-inferiority). Non-fatal myocardial infarction occurred in 2.9% of the patients receiving semaglutide and in 3.9% of those receiving placebo (HR: 0.74; 95% CI: 0.51–1.08; p=0.12); non-fatal stroke occurred in 1.6% and 2.7%, respectively (HR: 0.61; 95% CI: 0.38–0.99; p=0.04). Rates of death from cardiovascular causes were similar in the two groups. Rates of new or worsening nephropathy were lower in the semaglutide group but rates of retinopathy complications (vitreous haemorrhage, blindness, or conditions requiring treatment with an intravitreal agent or photocoagulation) were significantly higher (HR: 1.76; 95% CI: 1.11–2.78; p=0.02). Fewer serious adverse events occurred in the semaglutide group, although more patients discontinued treatment because of adverse events, mainly gastrointestinal. The authors concluded that in patients with Type 2 diabetes mellitus who were at high cardiovascular risk, the rate of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke was significantly lower among patients receiving semaglutide than among those receiving placebo, an outcome that confirmed the non-inferiority of semaglutide.8
An astonishing fact is the significant reduction of stroke events despite a minimal blood pressure reduction in the actively treated arm. As a risk of stroke is strongly dependent on the influence of hypertension or dysregulated haemodynamic control, further mechanistic studies to evaluate these aspects are called for, preferably by use of 24-hour ambulatory blood pressure monitoring and evaluation of central haemodynamics as well as arterial stiffness in the aorta. The increased risk of retinopathy is an unexpected finding that needs further evaluation as there is no real explanation at present, as a similar (non-significant) trend was also seen in the LEADER Study. The mean body weight in the semaglutide group, as compared with the placebo group, was 2.9 kg lower in the group receiving 0.5 mg and 4.3 kg lower in the group receiving 1.0 mg (p<0.001 for both comparisons).
EMPA-REG OUTCOME
Another class of anti-diabetic drugs are the SGLT2-inhibitors, promoting glucosuria. One member drug of this class, empagliflozin, was tested in a trial including patients with Type 2 diabetes mellitus at a high cardiovascular risk and then published in 2015.9 Patients were randomised to receive 10 mg or 25 mg of empagliflozin or placebo once daily. The primary composite outcome was death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke, as analysed in the pooled empagliflozin group versus the placebo group. The key secondary composite outcome was the primary outcome plus hospitalisation for unstable angina. A total of 7,020 patients were treated (median observation time, 3.1 years). The primary outcome occurred in 490 of 4,687 patients (10.5%) in the pooled empagliflozin group and in 282 of 2,333 patients (12.1%) in the placebo group (HR: 0.86; 95.02% CI: 0.74–0.99; p=0.04 for superiority). There were no significant between-group differences in the rates of myocardial infarction or stroke, but in the empagliflozin group there were significantly lower rates of death from cardiovascular causes (3.7% versus 5.9% in the placebo group; 38% relative risk reduction), hospitalisation for heart failure (2.7% and 4.1%, respectively; 35% relative risk reduction), and death from any cause (5.7% and 8.3%, respectively; 32% relative risk reduction). There was no significant between-group difference in the key secondary outcome (p=0.08 for superiority). Among patients receiving empagliflozin, there was an increased rate of genital infection but no increase in other adverse events. The effect on body weight was modest. The conclusion from the study was that patients with Type 2 diabetes mellitus at a high risk of cardiovascular events who received empagliflozin, as compared with placebo, had a lower rate of the primary composite cardiovascular outcome and of death from any cause when the study drug was added to standard care.9
In a further analysis from the EMPA-REG OUTCOME study of effects on renal protection, pre-specified renal outcomes included incident or worsening nephropathy (progression to macroalbuminuria, doubling of the serum creatinine level, initiation of renal replacement therapy, or death from renal disease) and incident albuminuria.10 The results indicated that incident or worsening nephropathy occurred in 525 of 4,124 patients (12.7%) in the empagliflozin group and in 388 of 2,061 (18.8%) in the placebo group (HR 0.61; 95% CI: 0.53–0.70; p<0.001). Doubling of the serum creatinine level occurred in 70 of 4,645 patients (1.5%) and in 60 of 2,323 (2.6%) in the placebo group, a significant relative risk reduction of 44%. Renal-replacement therapy was initiated in 13 of 4,687 patients (0.3%) in the empagliflozin group and in 14 of 2,333 patients (0.6%) in the placebo group, representing a 55% lower relative risk in the empagliflozin group. There were no significant between-group differences in the rates of incident albuminuria. The conclusion reached was that empagliflozin was associated with slower progression of kidney disease and lower rates of clinically relevant renal events than the placebo, when added to standard care.10
TREATMENT OF HYPERTENSION
Elevated blood pressure is a frequent companion to obesity and hyperglycaemia in Type 2 diabetes mellitus, as influenced by insulin resistance and associated abnormalities. The control of hypertension is one of the cornerstones of risk factor control in these patients, as stated in European guidelines from 2013.1,11 The goal for blood pressure control in these guidelines is set to <140/85 mmHg, with a corresponding goal of <140/80 mmHg (but <130/80 mmHg in specified subgroups) in USA guidelines from 2016.2 More recently a systematic review and meta-analysis has documented the importance of targeting patients with diabetes and a baseline systolic blood pressure ≥140 mmHg, but not to treat patients with a baseline systolic blood pressure <140 mmHg due to increased risk of myocardial infarction and cardiovascular mortality.12 This was challenged by another observational study from the Swedish National Diabetes Register (NDR) that showed a linear and increasing relationship between observed systolic blood pressure levels and risk of complications already from levels <120 mmHg in patients free from CVD.13 On the other hand, no specific analysis was made for patients already treated for hypertension and thus this observational study merely echoes the findings from an earlier observational publication from The UK Prospective Diabetes Study (UKPDS) with a similar design.14
Recently the publication of the SPRINT study in the USA has provoked a heated debate on blood pressure goals in hypertensive patients.15 Even if no patients with diabetes were included in SPRINT, arguments have been raised for implementing more strict blood pressure goals in patients with Type 2 diabetes mellitus, for example, the goal of <120 mmHg systolic blood pressure that proved more successful than the goal of <140 mmHg systolic blood pressure. On the other hand, the blood pressure methodology used in SPRINT was unusual and this could hamper any comparison with other similar intervention studies and also preclude from applying the findings in SPRINT when guidelines will be revised.16 In fact, the methodology used in SPRINT was to ask the patient to do a self-measurement of blood pressure while sitting alone in a room and using an automatic blood pressure device. This could have introduced bias as such a way to measure blood pressure could be 15–16 mmHg lower for systolic blood pressure as compared to office blood pressure recordings.17
Many experts thus think that current blood pressure goals for patients with diabetes should be kept, even if a minority of experts have argued for adopting the more stringent blood pressure goals from the SPRINT study.
TREATMENT OF HYPERLIPIDAEMIA
The cornerstone of lipid regulation in patients with Type 2 diabetes mellitus is still statins, used in effective dosages and based on a solid evidence base.18 The most effective statin is rosuvastatin and its preventative effects, as well as that of other statins, overshadows some other effects promoting glycaemia and increase of HbA1c.19 The mechanism behind this effect has been shown to be as a result of dual effects on glucose homeostasis by rosuvastatin, where insulin sensitivity is improved but beta cell function is impaired during in vitro experiments.20 Guidelines recommend a target for low-density lipoprotein (LDL) cholesterol to be <2.5 mmol/L for most patients with diabetes and <1.8 mmol/L for patients at a very high risk,1,2 for example, with a previous manifestation of a cardiovascular event motivating secondary prevention. Sometimes combination therapy, such as combining a statin with fibrates or cholesterol uptake inhibitors (ezetimibe), can also be an option.
An emerging and promising class of lipid-lowering drugs are the proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors,21 with a lowering effect on LDL cholesterol of about 60%. The first clinical outcome study is the FOURIER trial, expected to report in early 2017.22 FOURIER is a randomised, placebo-controlled, double-blind, parallel-group, multinational trial testing the hypothesis that adding evolocumab to statin therapy will reduce the incidence of major adverse cardiovascular events in patients with clinically evident vascular disease. The study population consists of 27,564 patients who have had a myocardial infarction, an ischaemic stroke, or symptomatic peripheral artery disease.22 If the clinical benefits dominate adverse effects this new class of drugs may prove to be beneficial in subgroups of patients with diabetes at very high risk, or who are statin intolerant.23 Recently, the GLAGOV study24 was presented where it was shown that among 968 patients with angiographic coronary disease treated with statins, addition of evolocumab, compared with placebo, resulted in a greater decrease in percentage of atheroma volume after 76 weeks of treatment. However, further studies are needed to assess the effects of PCSK9 inhibition on clinical outcomes.
SUMMARY
To protect patients with Type 2 diabetes mellitus from micro or macrovascular complications a wide and effective approach for overall risk factor control is recommended, as shown in the long-term follow-up of the Danish STENO-2 study.25 In this landmark study, it was concluded that at 21.2 years of follow-up after 7.8 years of intensified, multifactorial, target-driven treatment of Type 2 diabetes mellitus with microalbuminuria, there was a median of 7.9 years of gain of life. The increase in lifespan is matched by time free from incident CVD.
A rigid control of hypertension and hyperlipidaemia has long been recognised, and now three new large clinical trials have proven the benefits of glycaemic control by use of newer agents such as SGLT2 inhibitors (empagliflozin) or GLP-1 RAs (liraglutide, semaglutide). These drugs may also have some other, less well-defined beneficial effects on vascular and haemodynamic function, as manifested in successful reduction of congestive heart failure and stroke. Further studies should look more into the mechanisms explaining these beneficial effects that do not seem to be fully explained by weight reduction only.
In the future, the hope for a personalised ‘precision medicine’ bears hope also for the treatment of hyperglycaemia and risk factors in patients with Type 2 diabetes mellitus.26 However, sophisticated methods and new drugs cannot overcome an old but intrinsic problem in diabetes care; to increase the quality of the consultation based on understanding, support, and trust. A well-informed and motivated patient with Type 2 diabetes mellitus will most likely adhere to a healthy lifestyle and drug medication for risk factor control. Sadly, the opposite is often true in many fields of diabetes care and has to be improved based on a team approach in which physicians work together with diabetes nurses, dieticians, and other experts, but most importantly with the patient and his/her family within social networks to improve standards of care.