Abstract
In recent years the successful treatment of Type 2 diabetes mellitus through total calorific and/or dietary carbohydrate restriction has been well established. The use of low-carbohydrate diets for the adjunctive management of Type 1 diabetes mellitus has been studied but to a lesser extent. Over the past 20 years, a growing body of evidence has examined the effects of daily carbohydrate restriction on the key markers of glycaemic control, including blood glucose variability, average daily blood glucose readings, and HbA1c. The majority of publications to date have demonstrated a beneficial impact of carbohydrate reduction on glycaemic control. Indeed, similar findings have also been replicated using diets restricted to foods with a low glycaemic index. Interestingly, following a low-carbohydrate diet can also uncover the hyperglycaemic effects of fat and protein consumption, and the clinical implications of this will be discussed within this review. There is evidence, however, to suggest that these diets can be difficult to adhere to and that they may even pose health risks to the patient. Acutely, they can cause hypo or hyperglycaemic events, potentiate the risks of ketosis, and deplete systemic glycogen stores. The long-term effects of a low-carbohydrate diet are not well documented; however, possible complications can include alterations in lipid profiles, micronutrient deficiencies, cardiac complications, and nephrolithiasis. This review presents an overview of the major studies to date that have looked at carbohydrate dietary manipulation and the subsequent impact on glycaemic control in populations with Type 1 diabetes mellitus.
INTRODUCTION
In recent years much research has demonstrated the beneficial effects of low-carbohydrate and/or low-calorie diets on the clinical outcomes of patients with Type 2 diabetes mellitus (T2DM). Some of these regimes have shown a significant improvement, and occasionally pre-diabetic states approaching normoglycaemia have been achieved in certain individuals.1,2 The volume of studies examining carbohydrate intake manipulations in patients with Type 1 diabetes mellitus (T1DM) over the past 40 years have been more limited, but the results are promising.
The therapeutic use of low-carbohydrate diets in patients with T1DM has been used historically. Prior to the advent of medicinal insulin in the 1920s, management of this condition was via adherence to a strict low- carbohydrate intake or an intense fasting regime. The latter was hazardous, and patients often succumbed to emaciation or infection due to an undernourished immune system. However, just before the advent of parenteral insulin, promising results promoting careful adherence to a low-carbohydrate, high-fat diet emerged. These were even quoted as ‘staving off the emergence of severe acidosis.’3
This article reviews the studies to date that have investigated the use of carbohydrate manipulation to ameliorate glycaemic control in patients with T1DM. Additionally, the authors explore the challenges and the possible complications of following such a diet.
CARBOHYDRATES AND CIRCULATING GLUCOSE
Carbohydrates are one of the three macronutrients that are consumed in the human diet, with the other two being fat and protein. Carbohydrates can exist in several forms, including simple monosaccharides (e.g., glucose and fructose), disaccharides (e.g., sucrose and lactose), or in a polymeric form, sometimes known as complex carbohydrates (e.g., starch).4 Simple carbohydrates are very typically cheap and readily available, and it is unsurprising that they form an increasingly dominant proportion of processed food consumed in the developed world.5
The glycaemic load (GL) refers to the total amount of carbohydrate in any foodstuff, and by how much this will raise blood glucose (BG) levels. Additionally, the type of carbohydrate also affects postprandial glycaemic rise. The glycaemic index (GI) is a method of relatively ranking this change. Mono or disaccharides tend to have a higher GI, with complex carbohydrates having a lower GI.6 Consumption with protein or fat can lower the GI, leading to a more gradual postprandial rise in BG.7
METHODOLOGY
The authors of this article searched the databases of MEDLINE, Google Scholar, The Cochrane Library, and Web of Science for articles published between 1st January 1980 and 5th July 2018. Studies included were control trials, cohort studies, case-controls, cross-sectional studies, and case reports. Relevant review articles and meta-analyses were individually checked to ensure referenced studies were included. Participants were limited to T1DM patients following low-carbohydrate or low-GI diets. Outcomes were limited to the measures of glycaemic control (HbA1c, average daily BG, and BG variability).
LOW-CARBOHYDRATE AND LOW-GLYCAEMIC INDEX FOODS IMPACT ON GLYCAEMIC CONTROL
Dietary manipulation, specifically the adjustment of the GL of meals, has a significant influence on circulating BG levels thereafter.6 In the past 40 years, several studies have looked at how low-carbohydrate diets and/or low-GI diets affect glycaemic control in patients with T1DM. This review presents and discusses the clinical impact of these findings, with particular reference to low-carbohydrate diets (Table 1).8-16
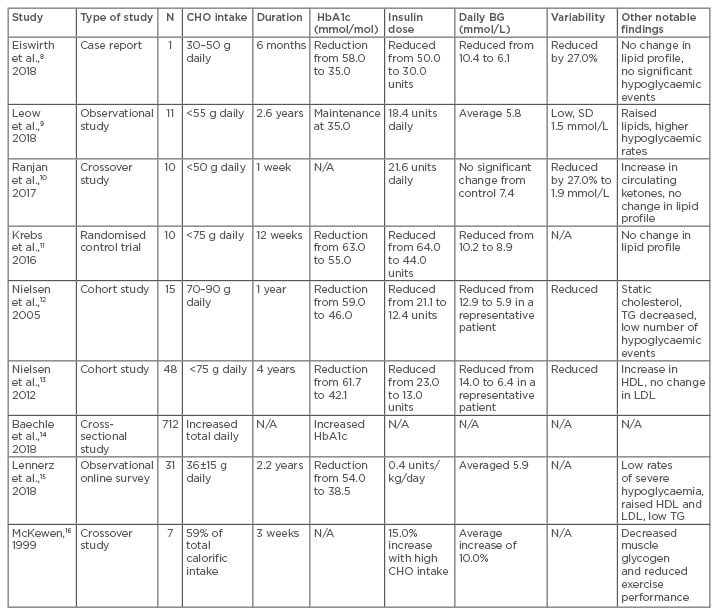
Table 1: Impact of altered dietary carbohydrate load on glucose homeostasis in patients with Type 1 diabetes mellitus.
BG: blood glucose; CHO: carbohydrate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; N/A: not applicable; SD: standard deviation; TG: triglycerides.
In 2018, Eiswirth et al.8 reported on the impact of a very low-carbohydrate diet (30–50 g) on average BG values, daily variability, and HbA1c in a patient over a 6-month period. They found a near normalisation of glycaemic indices (HbA1c: 34 mmol/mol; average daily BG: 6.1 mmol/L) with no significant hypoglycaemic episodes and minimal impact on circulating lipids. Although this was a case report limited to a single patient, the findings were further corroborated by a larger study, which examined 11 patients with T1DM over a 2–3-year period.9 Sustained consumption of <55 g carbohydrate daily demonstrated similar alterations in HbA1c levels (average HbA1c: 35 mmol/mol; average daily BG: 6.1 mmol/L) as well as reduced daily BG variability. However, the study showed an overall increased risk of hypoglycaemic episodes as well as an increase in non-high-density lipoprotein circulating lipids. The study was well designed and patients were followed-up for an average of 2.6 years. However, due to the low participant numbers the authors concluded that caution must be observed with the diet and larger scale studies were merited.
Ranjan et al.10 presented the short-term impact of either a high (>250 g) or low (<50 g) carbohydrate diet on glycaemic indices in a 2-week crossover trial. Their findings revealed similar average daily BG measurements between the two groups. In the low-carbohydrate diet group, significantly reduced BG variability was noted and, furthermore, a significantly higher proportion of the BG readings fell in the 3.9–10.0 mmol/L range compared to the high carbohydrate diet. It was a well conducted study, limited only by participant numbers (n=10). Another small trial in 2016,11 demonstrated significant HbA1c reductions (63–55 mmol/mol) in five patients following a carbohydrate restricted diet (average of 100 g) over 12 weeks. There were no adverse changes observed in BP, renal function, or lipid profiles. The study found that carbohydrate restriction required larger than predicted insulin doses to adequately control BG, which is discussed in a subsequent section of this review.
Nielsen et al.12 published an interventional study of 24 patients with T1DM, who followed a low-carbohydrate diet (70–90 g) for 1 year. They found significant reductions in HbA1c (58.5–46.4 mmol/mol), reduced BG variability, a nearly 6-fold reduction in hypoglycaemic events, a 16% reduction in serum triglycerides, and unchanged serum cholesterol levels. It was well conducted with significant findings, limited only by the small numbers, with data from 15 subjects used in the final analysis. These discoveries were replicated several years later by the same group who examined the impact of low carbohydrate intake on glycaemic control over 4 years on a group of 48 patients.13 Restriction to a daily carbohydrate intake of <75 g resulted in a sustained decrease of average HbA1c from 61.7 to 42.1 mmol/mol. Moreover, daily variability in BG readings was reduced as well. Interestingly, even patients only partly adherent to this diet over time sustained a reduction in HbA1c from an average of 59.6 to 51.9 mmol/mol. Conversely, patients who returned to a normal diet trended back to their average baseline HbA1c of 57.4 mmol/mol. The study gave a valuable observation into the impact of a low-carbohydrate diet on glycaemic variables, as well as providing extended follow-up that demonstrated the favourable longer-term dietary effects.
Beyond direct interventions, observational data have also demonstrated the beneficial impact of a low carbohydrate intake on glycaemic control in patients with T1DM. A nationwide survey of 712 11–19 year-olds with T1DM in Germany revealed a direct relationship between increased HbA1c and carbohydrate intake at breakfast and total daily carbohydrate intake.14 Additionally, an online survey of 316 patients following a low-carbohydrate diet (both adults and children) was published in 2018.15 Lennerz et al.15 found a normalisation of HbA1c (38.5 mmol/mol), with a low incidence of hospitalisation for ketoacidosis (1%) or hypoglycaemia (1%). The latter study did have several limitations, including an inability to confirm all medical data. Moreover, the study was pursued through an online social media group, which also increased the risk of selection bias.
Evidence further supporting that moderation of carbohydrate intake is beneficial for improved glycaemic control was further validated by a study over two 3-week periods that included seven males with T1DM.16 The subjects were grouped into a high carbohydrate diet group or a normal mixed diet group. Overall, a marked negative effect was noted with excessive carbohydrate intake. This caused a deterioration in glycaemic control (raised mean BG measurements), reduced exercise performance, and decreased glycogen storage.
Independent of the GL, low-GI diets can also improve the glycaemic control of patients, as demonstrated by an observational study by Queiroz et al.17 One hundred and forty-six patients (7–19 years old) were reviewed, and those who consumed either a lower carbohydrate (GL: <100) or lower GI (<55) diet had better glycaemic control than those who had a higher consumption of either high- carbohydrate or high-GI diets.
A publication by Giacco et al.18 investigated the long-term effects of a high-fibre, low-GI diet (average GI: 70%) versus a diet with an average GI of 90%. This was a large multicentre study that included 63 patients with T1DM. After 24 weeks, average daily BG, HbA1c measurements, and hypoglycaemic events were significantly lower in the low-GI diet group. Notably, the low-GI diet GI diet contained significantly more fibre (50 g versus 15 g), which may have had an influence on the improved glucose homeostasis. Reinforcing these findings, a small trial over 6 weeks found that a low-GI diet resulted in a more blunted postprandial BG rise following a carbohydrate challenge and also improved lipid parameters in a study of seven children.19
Even acute alterations in GI intake can have a marked influence on BG values. This was highlighted in a study of 23 paediatric patients, which examined the impact, in terms of glycaemic control, of alternating between a low-GI (<55) and a normal diet on 2 different days. The results were promising and revealed a 32% drop in average BG values on low GI days, as well as a reduction in the number of hyperglycaemic events.20 Another crossover paediatric trial of 20 patients in 2008 also found that low-GI diets can acutely reduce average daily BG readings from 10.2 mmol/L to 7.6 mmol/L, and reduce excursion out with the normal range. However, the study did demonstrate an increase in the frequency of minor hypoglycaemic events.21 Extrapolating this, a long-term trial with 104 children over 12 months found significantly lower HbA1c values in low-GI diets compared with carbohydrate exchange diets (64.5 versus 70.6 mmol/mol). Moreover, the low-GI diets resulted in fewer episodes of hyperglycaemia, better quality of life, and similar levels of hypoglycaemia.22
Further evidence in adults supporting low GI intake was presented by Lafrance et al.23 in 1998, who demonstrated that short-term intake of lower GI foods (GI of 66 versus 77) over 12 days was associated with lower daily average BG measurements in a crossover study of nine patients. Conversely, it is already known that consumption of high GI foods, such as cornflakes, cause a rapid rise in postprandial glucose,24 which can be more difficult to correct. However, low GI foods not only cause a gentler postprandial rise, but the BG area under the curve has been found to be up to 20% lower with lower GI food in macronutrient-matched meals.25 Such data highlight not only the importance of careful meal planning in terms of carbohydrate counting and load but also demonstrate that all carbohydrate sources are not equal in their net glycaemic effect.
Convincing large-scale observational data exist supporting the beneficial impact of low GI intake. A publication from the EURODIAB study of >2,800 patients with T1DM has shown that lower GI foodstuff consumption is linked to significantly lower HbA1c levels.26 Another publication analysed the same dataset and found higher carbohydrate intake to be associated with significantly increased HbA1c levels.27 Evidence further supporting these findings was published in a study of both patients with T1DM and T2DM in 2006.28 This study found that lower GI diets (25% lower mean GI) improved HbA1c levels by an average of 19%. There were similar findings in a meta-analysis of 14 randomised control trials examining the impact of low GI diet on T1DM and T2DM.29 The authors found an overall 7.4% reduction in HbA1c measurements with this dietary intervention.
Collectively, much evidence has shown the beneficial impact of low-GI or low-carbohydrate diets on key markers of stable glucose homeostasis. However, not all studies have supported the use of a low-carbohydrate or low-GI diet to improve glycaemic control, and indeed some have produced some conflicting evidence. The Diabetes Control and Complications Trial (DCCT) examined associations of nutritional intake, physiological parameters, and impact of daily activities on average HbA1c.30 The authors found that lower carbohydrate and higher fat intake were associated with higher HbA1c measurements. Furthermore, a paper published in 2015 studied 33 patients with T1DM, using linear regression models to compare nutritional intake and glycaemic indices.31 The authors demonstrated that increased carbohydrate intake was associated with greater periods of time spent in a euglycaemic state and less in a hyperglycaemic state. They postulated that the reason for this was that the patients were only correcting for glucose intake and were possibly not correcting the fat and protein content of their diet, thus creating a mismatch with the insulin dosing. This provides a credible explanation as to why, on occasion, low-carbohydrate diets can result in raised glycaemic indices.
In summary, a significant body of research from diverse study types has demonstrated that low-carbohydrate diets have the potential to reduce average HbA1c and BG variability in patients with T1DM. Low-GI diets have also shown clinical benefit; however, if an individual consumes large volumes of low GI food and increases their total GL then they risk negating the improvements in glycaemic control. In a similar manner, intake of high carbohydrate foodstuffs tends to cause a deterioration of glycaemic control. When consuming a low-carbohydrate meal, it would appear to be essential to also consider the impact of other macronutrients and correct insulin accordingly. Failure to do so may result in hyperglycaemia. The findings presented here must be taken with caution as larger, longer-term research is needed to explore the acute and chronic impacts of these diets. However, in order to pursue this several challenges and cautions should be addressed.
DIETARY CHALLENGES AND GLUCOSE HOMEOSTASIS
Modern diets are predominantly carbohydrate-rich. Worldwide guidelines differ regarding optimal target carbohydrate levels, but generally advise that carbohydrates should constitute from 45–65% of the total daily caloric intake.32 This equates to approximately 225–325 g carbohydrate per day, whereas a low-carbohydrate diet aims to restrict carbohydrates to <130 g per day and a very low-carbohydrate diet to usually <30–50 g per day.33 Such a dramatic reduction can be difficult, as it requires a good knowledge of food excipients, as well as careful meal planning on a daily basis. It may be restrictive in social situations, such as eating in restaurants or when consuming convenience food. Furthermore, there may be added financial costs, as many protein-rich foods (e.g., meat, fish, fowl, dairy) are more expensive than carbohydrate-rich alternatives (e.g., potato, rice, cereal, bread).34
Supporting patients through significant dietary change requires close input from dietitians, as well as the endocrinology and the primary care teams. This should be undertaken in a guided and transitory manner, facilitating incremental adjustments to lifestyle, cooking, and eating patterns over several weeks to months. Ideally, ketone levels should be monitored closely, as well as a personalised titration of insulin to nutritional intake to prevent hypo or hyperglycaemic events. Following a diagnosis of T1DM, patients are routinely taught by their clinical team how to match carbohydrate intake with insulin dosing for each meal;35,36 however, low-carbohydrate diets have a higher proportion of fat and protein content, which can also influence the pattern of postprandial glycaemic homeostasis.7
The evidence examining the effect of protein and fat on postprandial BG has been conflicting, but several recent studies have shown these macronutrients can independently and significantly impact BG. A study of 33 children by Smart et al.37 revealed an additive effect of both protein and fat to BG values 180–300 minutes post meal. Indeed, Paterson et al.38 demonstrated that an intake of 75–100 g of protein had a similar impact on BG measurements to 20 g carbohydrate after 240–300 minutes in control participants with T1DM. It was noteworthy that the postprandial increase in BG due to protein was more gradual compared to the rapid rise following carbohydrate intake. Although smaller doses of protein (21.5 g) have been found to have no appreciable effect on BG,39 severely restricting protein in the diet has been shown to cause a decrease in average daily BG by up to 30%, which was found to be mediated partly by reduced hepatic gluconeogenesis.40
In a recent case study, a patient with a daily carbohydrate intake <30 g regularly had to correct for fat and protein in her diet with larger boluses of insulin.8 This phenomenon showing that protein or fat can lead to postprandial glycaemic rises was also replicated by Krebs et al.11 and Uthoff et al.41 The latter paper studied 16 T1DM volunteers, and consistently found a rise in BG of an average of 2.2 mmol 4 hours after consumption of a fat and protein dominant meal. In line with this, dietary fat has also been shown to increase postprandial BG, independently of either carbohydrate or protein.42 The biochemistry underpinning these findings is complex; however, we know that glucose can be actively generated from protein catabolism through gluconeogenesis. It is not unreasonable to suggest that this process may be driven forward during a ketogenic diet and may partially explain the observed findings.
To address this phenomenon, two algorithms have emerged which help correct for consumption of these other macronutrients. These include the Warsaw Pump Therapy School formula,43 also known as the ‘Warsaw formula,’ and the Food Insulin Index method.44 They have both been found to decrease postprandial hyperglycaemic events; however, there is a potential increased risk of hypoglycaemia in the postprandial period with the Warsaw formula.43
CAUTIONS AND COMPLICATIONS
If a low-carbohydrate diet is adopted by an individual with T1DM, then several precautions should be acknowledged because clinical risks may exist. Acutely, alterations in biochemistry include risks of ketosis, hypo or hyperglycaemia, and glycogen depletion.9,30,31,45 With minimal carbohydrate intake, the body will increasingly catabolise protein and fat and become more dependent on circulating ketone bodies. In a normal individual these are unlikely to pose an acute health risk; however, in patients with T1DM they may contribute to an increased risk of ketoacidosis.46,47 On a more practical matter, as BG is generally lower due to decreased carbohydrate intake, there is the possibility of overcorrection when titrating insulin resulting in hypoglycaemia.
Another significant biochemical alteration can become more apparent, specifically the potential to deplete glycogen stores. This can blunt the physiological response to glucagon and contribute to hypoglycaemic mechanisms.48 It is essential, therefore, that when glucagon is required, supplementary carbohydrate should be administered as well, especially in patients following a reduced carbohydrate diet.
There are longer term risks to patients that low-carbohydrate diets may exacerbate. A review article published in 2016 explored these in detail, with specific attention to long-term complications, including growth alterations, hyperlipidaemia, nephrolithiasis, micronutrient deficiencies, and cardiac complications.47 Additionally, a series of six detailed case reports by de Bock et al.49 reinforced the risks associated with low-carbohydrate diets in children, including reduced growth velocity, increased risks of hypoglycaemia, micronutrient deficiencies, and dyslipidaemia. Collectively, these findings suggest caution is required in the use of such a diet in paediatric patients and may in theory be extrapolated to the adult population as well.
CONCLUSION
The emerging evidence supporting carbohydrate-based dietary modification for supplementary management of T1DM is indeed both controversial and compelling. In the short to medium-term it can lower daily BG variability and in certain cases even return HbA1c values to normal levels.8,9 Importantly, it is known that the amelioration of both of these parameters is associated with improved microvascular and macrovascular outcomes in patients with T1DM.50 However, this diet may be too restrictive or too difficult to adhere to for certain patients, and it is not without any adverse risks.47,49 These findings highlight the importance of a multidisciplinary approach in supporting patients wishing to pursue a low-carbohydrate diet, particularly providing guidance on how to follow such a diet in a safe and structured manner. The authors acknowledge that there are quantitative gaps in the literature that need to be addressed. Additionally, as insulin pumps can independently improve glycaemic control,51 low-carbohydrate dietary studies within this subgroup of patients also merits more detailed review. In conclusion, further research with larger scale studies conducted over an extended period are warranted to establish the long-term impact of low carbohydrate dietary manipulation on the overall health outcomes of patients with T1DM.








