Abstract
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9I) are a new class of medication that has recently arisen to combat hypercholesterolaemia. They are targeted towards patients who are unable to achieve low levels of low-density lipoprotein cholesterol despite maximum statin therapy, as well as those who are unable to tolerate maximum statin therapy due to side effects. Two of these medications were released in the summer of 2015: alirocumab and evolocumab. This article provides an overview of this medication class and analyses the clinical data from the numerous studies and trials conducted on both of these medications for their efficacy and safety outcomes. Data indicate that PCSK9I are both a safe and effective means of lowering low-density lipoprotein cholesterol levels of resistant or otherwise currently unmanaged hypercholesterolaemia patients.
INTRODUCTION
Atherosclerosis can be considered a metabolic disease and the clinician needs to realise this and consider cardiovascular disease prevention. Low-density lipoprotein cholesterol (LDL-C) is a causal risk factor for atherosclerotic cardiovascular disease (ASCVD) and statins represent the most commonly prescribed class of LDL-C-lowering medications. However, despite attempts at adequate pharmacologic treatment, acceptable LDL-C levels cannot be achieved in certain patients due to statin intolerance or ineffectiveness. These gaps in patient coverage have led to the development of additional therapies to improve treatment. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (PCSK9I) are quickly rising to the occasion, providing benefit to those neglected patients in clinical trials.
PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9
PCSK9 is an important serine protease involved in the regulation of LDL-C metabolism. Hepatocytes are the predominant site for PCSK9 production.1 Expression of PCSK9 and LDL receptors (LDLR) are closely regulated by sterol regulatory element-binding protein-2 and intracellular cholesterol.2 PCSK9 circulates in three forms: a PCSK9 monomer, LDL-C-bound PCSK9, and a 55 kDa furin-cleaved inactive fragment.3 PCSK9 acts both intracellularly (as a chaperone rather than a catalytic enzyme) as well as a secreted factor.3,4 Extracellular PCSK9 binds to epidermal growth factor-like repeat-A located at the extracellular domain of the LDLR and interferes with the LDLR, recycling back after internalisation with LDL-C and directing the LDLR to the lysosomes for its destruction.5
Gain-of-function mutations alter the natural function, leading to hyperactivity of the PCSK9 gene, which degrades LDLR in excess, thus increasing LDL-C levels.6-8 These rare mutations, recognised as a third cause of autosomal-dominant familial hypercholesterolaemia (FH), are not only associated with high cholesterol levels but also with a greater atherosclerotic burden.6-9 Conversely, loss-of-function mutations result in lower LDL-C levels throughout the course of life and a lower rate of cardiovascular events.10,11 Therefore, these findings definitively established PCSK9 synthesis, activity, and/or the PCSK9–LDLR binding mechanism as a therapeutic target for reducing LDL-C and the risk of ASCVD.
PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9 INHIBITORS
Various modalities to inhibit PCSK9 have been studied, including inhibition of production by gene silencing through antisense oligonucleotides or small interfering RNA; prevention of PCSK9 binding to LDLR using monoclonal antibodies (mAb), epidermal growth factor-like repeat-A, mimetic peptides, or adnectins; and inhibition of PCSK9 autocatalytic sites.12-15
Inclisiran is a long-acting RNA-interference agent targeting PCSK9 synthesis. A Phase I trial16 demonstrated reductions of 75% and 51% in PCSK9 and LDL-C concentrations, respectively, with doses ≥300 mg. In a Phase II randomised clinical trial (RCT),17 including adults unable to reach LDL-C goals with maximum-tolerated statin doses, the mean reductions in LDL-C levels were 27.9–41.9% after a single dose of inclisiran and 35.5–52.6% after two doses (p<0.001 for all comparisons versus placebo) at Day 180. At Day 240, the reductions in PCSK9 and LDL-C remained significantly lower than baseline with all the studied doses. Two 300 mg injections produced the greatest reduction in LDL-C, with 48% of patients achieving an LDL-C level <50 mg/dL. Serious adverse events (AE), tracked until Day 210, occurred in 11% of inclisiran recipients and 8% of placebo recipients. Injection-site reactions, the most common AE, occurred in 5% of inclisiran recipients but in no placebo recipients. Injections were given every 3 or 6 months. The results of this study will inform the dose and dosing regimen for a Phase III cardiovascular outcomes study with inclisiran.
Use of mAb has been the most efficacious approach thus far in inhibiting PCSK9. Systemic absorption of PCSK9-specific mAb occurs via lymphatic circulation and via diffusion to blood vessels in proximity of the injection site.18,19 The time required to reach the peak of maximal concentration varies between 2 and 8 days, with absolute bioavailability that ranges from 50–100%.20 Indeed, LDL-C levels decrease rapidly, reaching their lowest concentrations by approximately 15 days. With time, levels of plasma mAb decrease, reaching undetectable levels by around 60 days.21 Unlike small molecule drugs, which are commonly eliminated via renal or hepatic routes, mAb are cleared by different mechanisms, including fluid-phase pinocytosis and receptor-mediated endocytosis in phagocytes.18,22 However, no additional information has been provided to fully understand the mechanisms of Ab internalisation and clearance.
Currently, at least six mAb have been or are being developed and tested. Alirocumab and evolocumab are the front-runners of the PCSK9I that are approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) to be used in adult patients with heterozygous FH and non-FH or clinically significant ASCVD requiring additional LDL-C lowering or mixed dyslipidaemia, as adjunct treatment to diet. These include the following cases:
- In combination with a statin or a statin with other lipid-lowering therapies (LLT) in patients unable to reach LDL-C goals with maximum-tolerated statin doses.
- Alone or in combination with other LLT in patients who are statin-intolerant or for whom a statin is contraindicated.
Evolocumab reduced LDL-C by approximately 30% in a patient subgroup that had at least one mutant LDLR allele with residual functionality, whereas individuals with two null or completely non-functional alleles did not respond.23,24 Therefore, evolocumab has received an additional indication in homozygous FH adults and adolescents >12 years old with residual LDLR function in combination with other LLT.
The starting dose of subcutaneously injected alirocumab is 75 mg biweekly; it can be increased up to 150 mg biweekly. In comparison, the dose of evolocumab is 140 mg biweekly or 420 mg monthly. The global clinical development programme for bococizumab, a humanised, rather than fully human, mAb with approximately 3% murine sequence remaining in the antigen-binding complementarity-determining regions, was discontinued due to an unanticipated attenuation of LDL-C lowering over time, a higher level of immunogenicity, and a higher number of injection-site reactions.
OVERVIEW OF CLINICAL TRIALS OF PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9: SPECIFIC MONOCLONAL ANTIBODIES
To date, almost 30 Phase II and III RCT have been reported, mainly using evolocumab or alirocumab versus various comparators. Patient groups studied included those with FH, hypercholesterolaemia with high cardiovascular risk on usual or maximal LLT, and statin intolerance.
Efficacy of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Long-Term Trials (Table 125-31)
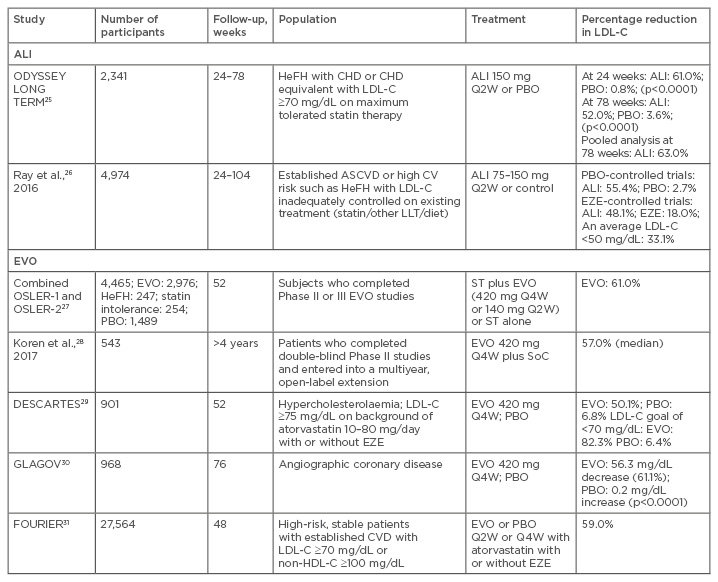
Table 1: Efficacy of anti-proprotein convertase subtilisin/kexin type 9 antibodies in patients with hypercholesterolaemia: summary of Phase III and long-term trials selected for inclusion of review of data.
ALI: alirocumab; ASCVD: atherosclerotic cardiovascular disease; CHD: coronary heart disease; CV: cardiovascular; CVD: cardiovascular disease; EVO: evolocumab; EZE: ezetimibe; HeFH: heterozygous familial hypercholesterolaemia; LDL-C: low-density lipoprotein cholesterol; LLT: lipid-lowering therapy; PBO: placebo; Q2W: one dose every 2 weeks; Q4W: one dose every 4 weeks; ST: standard therapy; SoC: standard of care.
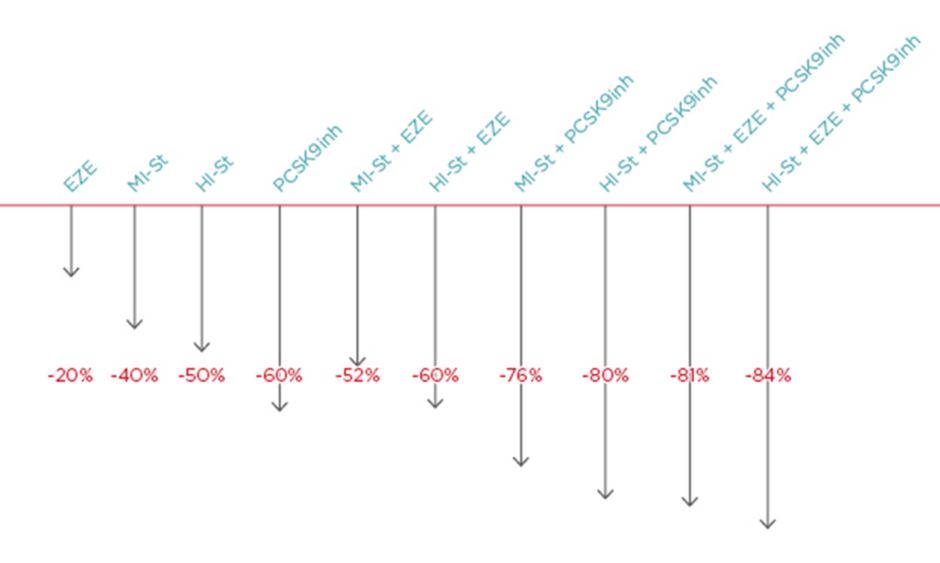
ODYSSEY LONG TERM25 was a Phase III RCT comparing the efficacy of alirocumab with placebo for 78 weeks in patients at high ASCVD risk. At Week 24, there was a 61.0% reduction from baseline in LDL-C levels in the alirocumab group compared to a 0.8% increase in the placebo group (62.0% reduction in alirocumab group compared to placebo) (p<0.0001). The difference between alirocumab and placebo in LDL-C reduction at Week 24 was similar in patients with heterozygous FH and non-FH. The LDL-C at Week 48 in alirocumab versus placebo groups was 57.9 mg/dL versus 122.6 mg/dL, respectively. Alirocumab therapy sustained a 58.0% reduction in LDL-C at 78 weeks. Eighty-one percent of alirocumab patients achieved their prespecified LDL-C goal compared to 9% for placebo (p<0.0001).
Data from the open label OSLER-1 and OSLER-2 RCT assessing the efficacy of evolocumab were combined into a single analysis set.27 After a median follow-up of 11.1 months, evolocumab reduced LDL-C from a mean of 120 mg/dL to 48 mg/dL and patients achieved a 61.0% reduction in LDL-C compared to standard therapy alone (p<0.001). Significant reductions in lipoprotein (Lp)a and triglyceride levels with a mild increase in high-density lipoprotein cholesterol (HDL-C) with treatment of either evolocumab or alirocumab were also noted.25,27 Evolocumab reduced non-HDL-C levels by 52.0% and apolipoprotein B (apoB) by 47.3% (p<0.001).27 Results from an open-label extension (OLE) study showed sustained reductions in LDL-C levels. At approximately 2, 3, and 4 years of follow-up, the median LDL-C concentration was reduced by 59.0%, 59.0%, and 57.0%, respectively, from parent study baseline.28
Pooled Analyses and Meta-Analyses
Data were pooled from 10 Phase III ODYSSEY trials, including patients randomised to alirocumab 75/150 mg every 2 weeks or control for 24–104 weeks and added to background statin therapy in 8 trials.26 Six of the studies, representing ˜80% of the population, had a minimum study duration of 52 weeks. The average percentage change in LDL-C from baseline was -55.4% for alirocumab and 2.7% for placebo, and -48.1% with alirocumab and -18.0% with ezetimibe. Overall, 33.1% of patients achieved an average LDL-C <50 mg/dL during treatment (44.7–52.6% allocated to alirocumab, 6.5% allocated to ezetimibe). The overall distribution of each lipid parameter during treatment largely reflected the greater proportion of patients achieving very low levels of LDL-C, non-HDL-C, and apoB in the alirocumab group.
Three meta-analyses confirmed the efficacy of PCSK9I (Table 2).32-34 Zhang et al.32 demonstrated that for equipotent dosages and dosing intervals, evolocumab and alirocumab had essentially identical LDL-C-lowering efficacy at 52 weeks follow-up. The LDL-C reduction following evolocumab treatment was 54.6%, and the absolute mean reduction was -78.9 mg/dL versus placebo and -36.3% versus ezetimibe. HDL-C increased by 7.6% versus placebo and 6.4% versus ezetimibe. The efficacy outcome for alirocumab was similar. LDL-C reduced by >50% versus placebo. A less marked reduction in LDL-C was found when compared with ezetimibe (-29.9%). HDL-C level increased by a mean of 8%. Besides a significant reduction in non-HDL-C, there was a reduction in very-LDL-C with evolocumab and in apoB with alirocumab.32 Lipinski et al.33 reported that PCSK9 inhibition alone led to 57.0% lower LDL-C, 46.0% lower apoB, and 24.3% lower Lpa. Compared with placebo, PCSK9I resulted in a LDL-C reduction of 69.0%. A maximum reduction of LDL-C of 57.8% was seen in patients with high baseline statin therapy. Many trials found at least one LDL-C value as low as 25 mg/dL.
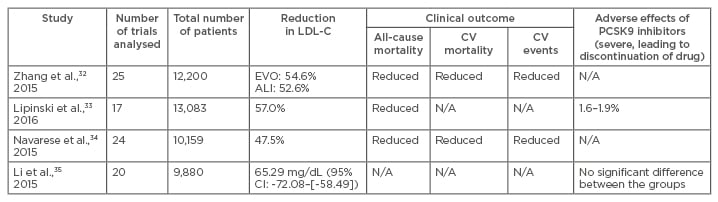
Table 2: Comparison of four meta-analyses on proprotein convertase subtilisin/kexin type 9 inhibitors.
ALI: alirocumab; CI: confidence interval; CV: cardiovascular; N/A: data not available; LDL-C: low-density lipoprotein cholesterol; PCSK9: proprotein convertase subtilisin/kexin type 9.
Navarese et al.34 conducted a meta-analysis of 24 RCT with a mean follow-up of 44.6 weeks. Most trials involved patients treated with statins who had not met target LDL-C goals, although some focussed only on statin-intolerant patients. Data showed that PCSK9I led to a 25.0% average reduction in Lpa and a 47.5% reduction in LDL-C (p<0.001), with a larger reduction when compared with placebo (-58.8%) than when compared with ezetimibe (-36.2%). The relative reduction in LDL-C levels with PCSK9I was similar to, or even slightly greater, in statin-treated patients. Although statins might induce PCSK9 upregulation, partially attenuating the LDL-C-lowering effect of PCSK9I, a combination of statins and mAb results in an additional reduction of LDL-C by 50–60% compared to statin monotherapy; this finding highlights the potential value of PCSK9 inhibition as an adjunct to standard therapy.36,37 In addition, the effect was measured independent of the applied statin doses.29 Zhang et al.38 found that PCSK9-specific mAb reduced LDL-C in combination with a statin and suppressed hepatocyte sterol regulatory element-binding protein-regulated genes. Li et al.35 reported similar efficacy of evolocumab and alirocumab in absolute terms (Table 2). Since other meta-analyses evaluated efficacy in relative terms, this meta-analysis could not be compared directly; however, the overall results were consistent with studies reporting percent changes.
Recently, a pooled analysis focussing on elderly patients demonstrated that evolocumab-treated patients ≥65 years achieved an LDL–C reduction of 58.4–62.9% at the mean of Weeks 10 and 12.39 The LDL–C concentration in patients ≥75 years was reduced by 59.9–68.6%.
Safety and Adverse Events of Proprotein Convertase Subtilisin/ Kexin Type 9 Inhibitors (Table 3)
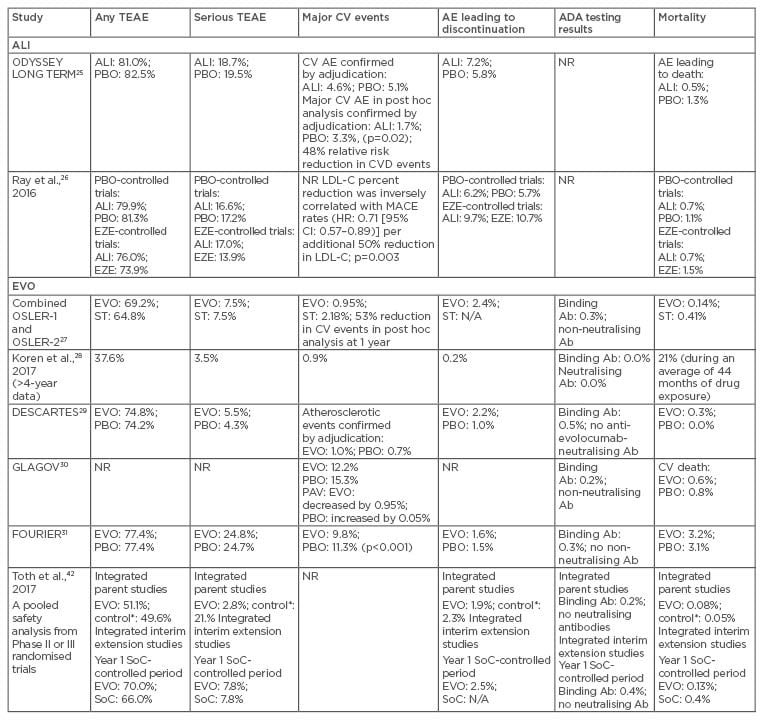
Table 3: Safety of anti-proprotein convertase subtilisin/kexin type 9 monoclonal antibodies in patients with hypercholesterolaemia.
*Control includes placebo and ezetimibe treatment groups.
Ab: antibody; ADA: antidrug antibody; AE: adverse event; ALI: alirocumab; CI: confidence interval; CV: cardiovascular; CVD: cardiovascular disease; EVO: evolocumab; EZE: ezetimibe; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; MACE: major adverse coronary events; N/A: not applicable; NR: not reported; PAV: percent atheroma volume; PBO: placebo; SoC: standard of care; ST: standard therapy; TEAE: treatment-emergent adverse event.
In the ODYSSEY LONG TERM trial,25 overall AE rates were similar among the groups. Myalgia was more frequent with alirocumab than with placebo (5.4% versus 2.9%; p=0.006). Injection-site reactions and neurocognitive and ophthalmologic events were also more frequent in the alirocumab group, but differences did not reach statistical significance. Discontinuation due to AE was comparable among groups. Of interest, AE in the subgroup of patients with a LDL-C level <25 mg/dL were similar to the overall alirocumab group.
In the OSLER trials, most AE occurred with almost similar frequency in all groups.27 Injection-site reactions led to 0.2% of patients stopping therapy. Arthralgia, headache, limb pain, and fatigue were more frequent in the evolocumab group, but liver function and creatine kinase remained unchanged. Rates of overall AE, serious AE, and elevations in aminotransferase or creatine kinase levels were similar among evolocumab-treated patients with LDL-C levels <40 mg/dL or <25 mg/dL as in those with higher levels. A total of 79% of patients persisted with evolocumab treatment, with a mean exposure duration of 44 months.28
Four meta-analyses confirmed the safety of PCSK9I, without differences regarding serious AE among patients treated with both mAb and placebo or standard therapy.32-35 Zhang et al.32 showed that for equipotent dosages and dosing intervals, evolocumab and alirocumab had essentially identical side effect profiles. Overall, no significant difference between PCSK9I and placebo (or ezetimibe) was noted, except that alirocumab was associated with an increased rate of injection-site reactions (p=0.02) and evolocumab reduced the rate of abnormal liver function (p=0.03), both compared with placebo. No significant difference in safety outcomes was detected between monthly 420 mg and fortnightly 140 mg evolocumab treatments.
In a pooled analysis of alirocumab trials up to 78 weeks in duration, overall rates of treatment-emergent AE (TEAE), serious AE, discontinuations because of TEAE, and deaths were comparable with controls.40 Alirocumab was associated with a higher incidence of local injection-site reactions, pruritus, and upper respiratory tract infection signs and symptoms. As patients with persistently positive antidrug antibody (Ab) have been shown to experience a greater incidence of injection-site reactions, an immune-based response is possible; however, the precise mechanism is unknown.41 Similarly, the precise mechanism for the increased rate of pruritus with alirocumab is unknown. Jones et al.40 considered the higher incidence of upper respiratory tract infection signs and symptoms to be a chance finding. It was not related to low LDL-C concentrations and there was no mechanistic basis for an increase in this AE category with alirocumab. The incidence of musculoskeletal, neurologic, ophthalmologic, and hepatic events was similar between alirocumab and control groups.
Similarly, a pooled safety analysis of evolocumab in >6,000 patients from double-blind and OLE studies demonstrated comparable overall AE rates between evolocumab and control in parent trials and in Year 1 of OLE trials, as were those for serious AE.42 Nasopharyngitis was the most common AE documented. Injection-site reactions were reported by 3.3% of patients receiving evolocumab and 3.0% of controls in the parent studies and by 4.1% in the extension studies. Infrequent elevations of serum transaminases, bilirubin, and creatine kinase, as well as muscle-related AE, occurred, with similar rates between groups. Neurocognitive AE were infrequent and balanced during the parent studies. In the OLE trials, 0.9% of evolocumab-treated patients and 0.3% of controls reported neurocognitive AE.
No new safety concerns were identified in the GLAGOV trial.30 The study was not large enough to make any definitive statements about safety, but AE looked reassuring, with no significant excess in rate of injection-site reactions, myalgia, and neurocognitive events. The rates of laboratory abnormalities were low in both groups. The FOURIER study31 found no significant difference between the groups with regard to AE, with the exception of injection-site reactions, which were more common with evolocumab (2.1% versus 1.6%).
Muscle-related AE occurred in 12% of evolocumab-treated patients and 23% of ezetimibe-treated patients in the 12-week, Phase III GAUSS-2 trial evaluating statin-intolerant patients.43 The percentage of patients who discontinued participation in the study was similar among patients receiving evolocumab and ezetimibe.44 The Phase III ODYSSEY ALTERNATIVE trial44 included patients with a history of muscle symptoms related to at least two previous statins. This was the only study design to include a placebo run-in period and a statin-rechallenge to confirm statin intolerance. The run-in period was completed by 87.0% of patients, with 15.9% of alirocumab-treated patients discontinuing due to muscle-related symptoms. The rate of skeletal muscle-related TEAE was significantly lower for alirocumab-treated patients than for atorvastatin-treated patients.45
A potential problem of long-term Ab treatment is the occurrence of antidrug Ab. Humanised mAb are immunogenic and can trigger production of neutralising antibodies. Approximately 16% of patients taking bococizumab develop high titres of neutralising Ab that interfere with the capacity of the Ab to bind to PCSK9, resulting in marked attenuation of its LDL-C-lowering effect. Evolocumab and alirocumab are fully human mAb and, therefore, are theoretically less likely to induce auto-Ab compared to humanised (95% human sequence) therapeutic Ab. Very few cases of antidrug Ab have been published to date. Only 0.2–0.3% of patients developed transient anti-evolocumab Ab.28,37 No PCSK9-neutralising Ab were observed.27,28,30,42 No reduction of LDL-C-lowering or an off-target effect has been reported, but this topic requires long-term observation.42
PCSK9 inhibition has been suggested to have a deleterious impact on adrenal function as a result of impaired delivery of Lp cholesterol to the adrenal glands to support adrenal steroidogenesis, particularly when LDL-C is reduced to extremely low levels. Evaluation of a male patient with a heterozygous PCSK9 loss-of-function mutation provided the opportunity to determine the effect of PCSK9 deficiency on adrenal function.46 The patient had no detectable plasma PCSK9 and an LDL-C level of 24 mg/dL. Baseline adrenal function tests and cortisol response to adrenocorticotropic hormone stimulation were normal, suggesting that genetic PCSK9 deficiencies were not associated with abnormal adrenal function. Moreover, the safety of PCSK9 inhibition was evident in a family, including the proband with an LDL-C of 49 mg/dL and a daughter with an LDL-C of 14 mg/dL. The daughter had a premature stop codon in the protein transcript inherited from her mother and a second mutation from her father that deleted an arginine residue at codon 97. She was reported to have total deficiency of PCSK9 but normal intelligence, motor skills, kidney and liver function, and blood pressure.47
An analysis including Phase II and III trials showed that alirocumab was associated with a favourable safety profile when LDL-C was reduced to very low levels.48 Of alirocumab-treated patients, 23.8% achieved LDL-C <25 mg/dL and 8.6% achieved LDL-C <15 mg/dL. No safety signals were observed and TEAE were generally similar among all alirocumab-treated patients, those achieving very-LDL-C levels, and those in the control group.
Since cholesterol is an important component of neurons and PCSK9 is involved in cortical neuron regeneration, impairments of neurocognitive function have been a major concern of the drastic LDL-C reduction following PCSK9I administration.49 However, PCSK9 loss-of-function variants have not been associated with impaired cognitive performance.50 In addition, Lp and mAb do not cross the blood–brain barrier and there is even some evidence of a possible decrease in dementia risk.51 In the ODYSSEY LONG TERM and OSLER studies, neurocognitive changes were more common with PCSK9I (although not statistically significant) but were infrequent (<1%) and not related to the degree of LDL-C reduction.25,27,28 However, these studies have several limitations: the occurrence of AE may have been confounded by the open-label design of the OSLER studies; the number of patients is relatively small and the duration of follow-up is too short to be considered definitive; and there is no information about the patients’ baseline cognition. Neurocognitive events were self-reported by patients and no formal neurocognitive testing was used.
Navarese et al.34 did not provide any neurocognitive AE data. A network meta-analysis found a significant 2.3-fold increased risk of neurocognitive AE with PCSK9I, primarily because of the inclusion of the aforementioned two trials.25,27,33 The most common events were memory loss and amnesia (both ≤0.5%).40 Serious neurologic events were single occurrences, without evidence of a common pathogenic mechanism. In contrast, a pooled analysis did not find any neurocognitive event differences between alirocumab and placebo or ezetimibe.40 A more objective assessment of evolocumab’s neurocognitive effects was recently completed in the EBBINGHAUS study, showing no differences between groups with regard to executive function as well as working memory, memory function, and psychomotor speed after 20 months.52 It should also be noted that LDLR can act as the entry point for some viruses, including hepatitis C virus,53 and it is unknown whether PCSK9I can increase the risk of hepatitis C virus infection.
CONCLUSION
PCSK9I have satisfactory lipid-modifying effects with acceptable safety profiles in patients with FH or non-FH. Some issues, however, should be taken into consideration. First, the mAb is injected subcutaneously, making it less possible for a long-term treatment. Additionally, caution needs to be taken for possible hypocholesterolaemia-associated AE and antidrug Ab. More clinical data are needed to ascertain whether these are potential problems or are of no concern.