Abstract
Chorioretinal vascular diseases are among the leading causes of blindness in industrialised countries. The recent development and widespread adoption of intravitreal pharmacotherapy enables surgeons to not only stabilise disease in most cases, but also improve visual acuity (VA). Inhibitors of vascular endothelial growth factor (VEGF) have become first-line therapy for patients with neovascular age-related macular degeneration (nAMD), diabetic macular oedema (DMO), and oedema due to retinal vein occlusions (RVO). The pivotal Phase III registration studies evaluated the efficacy and safety of monthly or bimonthly injections of anti-VEGF drugs, and remain the standard against which other treatments and injection regimens are compared. Adhering to a regimen of monthly drug injections requires considerable patient compliance and allocation of substantial healthcare resources, therefore most physicians use individualised treatment strategies. As-needed (PRN) and treat and extend (T&E) regimens reduce the number of clinic visits, intravitreal injections, or both, and are less expensive than monthly therapy. Both regimens reduce unwanted macular oedema and improve VA, but compared to monthly therapy over the course of 1 year, may be 1–3 letters less effective. Trials of 5-year duration suggest that PRN treatment modulates the severity of diabetic retinopathy (DR) and stabilises vision in patients with DR. Long-term data comparing these strategies in patients with nAMD and RVO are lacking, but VA frequently declines when observation periods and treatment intervals are extended beyond 4 weeks. Current observations suggest that aggressive long-term therapy with frequent injections may produce the best VA results in patients with nAMD and RVO.
INTRODUCTION
Chorioretinal vascular conditions, neovascular age-related macular degeneration (nAMD), diabetic retinopathy (DR), and retinal vein occlusions (RVO), are among the leading causes of irreversible blindness in industrialised countries.1 Laser photocoagulation was the standard of care for decades, but significant post-treatment improvements in visual acuity (VA) were unusual, therefore laser techniques were performed primarily to stabilise vision.2 Recent advances in our understanding of the pathophysiological mechanisms responsible for vision loss have focussed on the pivotal role of vascular endothelial growth factor (VEGF).
VEGF regulates the integrity of the blood-retinal barrier and plays a critical role in angiogenesis. New blood vessel growth in nAMD damages the photoreceptor/retinal pigment epithelium/choriocapillaris complex and diminishes VA. Neovascularisation in diabetic eyes with widespread retinal ischaemia causes profound vision loss due to vitreous haemorrhage and traction retinal detachments. Elevated VEGF levels cause breakdown of the blood-retinal barrier in eyes with non-proliferative DR and RVO, and leads to the accumulation of macular oedema. Understanding VEGF biochemistry has enabled us to develop potent pharmacotherapies that reduce the damaging effects of angiogenesis.3 Drugs that bind diffusible VEGF have become first-line therapy for the treatment of these conditions.
Ranibizumab (Lucentis®, Genentech, California, USA/Roche, Basel, Switzerland) and aflibercept (Eylea®, Regeneron, New York, USA) are approved for the treatment of nAMD and macular oedema due to DR and RVO, and off-label bevacizumab (Avastin®, Genentech/Roche) is used by most USA retinal specialists.4 Phase III registration trials that evaluated monthly (ranibizumab and aflibercept) or bimonthly (aflibercept, after a 3–5-month induction sequence) regimens produced significant VA improvements and disease stabilisation in most patients.5-14 Table 1 details the evidence that supports the use of each drug. Adhering to these treatment regimens challenges most patients and physicians find their clinics filled with re-injections. As a result, most physicians have switched to as-needed (PRN) or treat and extend (T&E) regimens that reduce the number of injections, clinic visits, or both (Figure 1).15
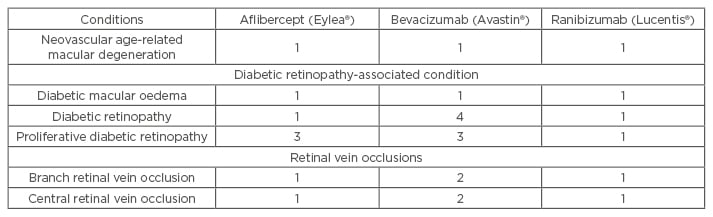
Table 1: The level of clinical evidence that supports the use of aflibercept, bevacizumab, and ranibizumab for the treatment of the most common chorioretinal vascular conditions.
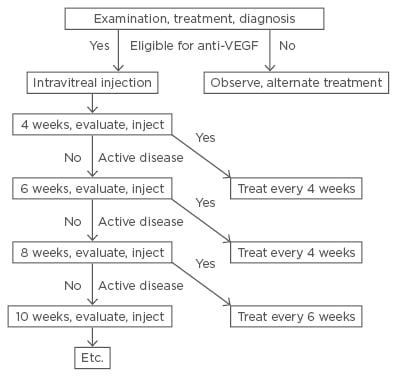
Figure 1: Flow chart showing the time-related work flow for the treat and extend strategy.
Patients receive intravitreal injections at every clinic visit. If the disease is inactive, the interval to the next visit is extended by 2 weeks (left side of figure). If the disease is active, the interval to the next visit is shortened by 2 weeks (right side of figure) and the patient’s subsequent return visits are scheduled according to this interval.
VEGF: vascular endothelial growth factor.
The benefits accrued with less intensive, individualised treatment regimens are not without associated drawbacks, which must be considered by physicians and patients when choosing a strategy. This manuscript discusses many of the advantages and disadvantages of individualised treatment regimens.
NEOVASCULAR AGE-RELATED MACULAR DEGENERATION
Pegaptanib (Macugen®, Ophthotech, New York, USA) was the first drug approved (2004) for the treatment of nAMD and physicians were instructed to inject it at 6-week intervals.16 Because patients lost a mean of -7 letters during the first year of treatment, pegaptanib was abandoned by most retinal specialists before individualised strategies could be studied. Bevacizumab, a full-length, recombinant, humanised antibody against VEGF was approved for the intravenous treatment of solid tumours (2004) before being used off-label for nAMD and RVO in 2005.17,18 Bevacizumab use spread rapidly throughout the world, making it most retinal specialists’ first choice in anti-VEGF therapy. Ranibizumab, a recombinant, humanised, antibody fragment, received US Food and Drug Administration (FDA) approval in 2006 based on results of the pivotal, 2-year, Phase III ANCHOR and MARINA trials.5,6 Patients with classic choroidal neovascular membranes (CNVM) in ANCHOR improved by a mean of +11 letters,5 whereas those with occult CNVM in MARINA improved by a mean of +7 letters.6 Patients enrolled into the 2-year HORIZON extension trial lost a mean of -7 letters while receiving ranibizumab quarterly PRN.19 A smaller number were examined and treated at the physician’s discretion in the SEVEN-UP extension trial. At a mean of 7.3 years after entry into ANCHOR and MARINA, only 43% of patients had stable or improved vision from the original baseline and mean VA loss in this cohort was -8.6 letters.20
Patients in other long-term AMD studies appear to have fared somewhat better. Gillies et al.21 followed 1,212 patients for a mean of 53.5 months and 45% of them for at least 60 months. Mean VA improved by +6.3 letters at Month 6, remained above baseline for 6 years, but fell -2.6 letters below baseline by Year 7 in the 131 remaining eyes. Participants received a mean of 5 injections/year after Year 2, at least twice as many as those in HORIZON, and 3-times as many as in the 7-UP extension (31%).
The best long-term results came from a retrospective study of 109, 75, and 45 patients who were aggressively treated with anti-VEGF drugs (mean of 10.5 injections/year) at fixed intervals of 4–8 weeks, for 5, 6, and 7 years, respectively.22 Mean VA improvements at these time points were +14.0, +12.2, and +12.1 letters, and 43.2% of eyes achieved a final VA of 20/40 or better. VA improvement peaked at +16.1 letters at Year 2, after which vision declined by an average of 0.8 letters/year. Each of these studies featured individualised therapy for most of the period but visual results correlated strongly with more intensive therapy.
Several multicentre, randomised, national trials, such as CATT in the USA,23 IVAN in the UK,24 MANTA in Austria,25 GEFAL in France,26 BRAMD in the Netherlands,27 and LUCAS in Norway,28 showed that ranibizumab and bevacizumab produce similar results when evaluated head-to-head. Combined data from CATT and IVAN showed that patients receiving monthly injections improved by a mean of +2.2 letters more than those treated PRN.24 Patients treated with a T&E strategy for 12 months in LUCAS improved by +8.0 to +8.2 letters, similar to results from trials that featured monthly injections.28
Aflibercept, a recombinant fusion protein with native-receptor VEGF-binding sequences,29 a very high VEGF binding affinity,30 and favourable pharmacokinetic profile, was approved for the bimonthly treatment of nAMD after a 3-injection induction sequence. In the VIEW trials, 1-year VA improvements of +8.3 to +9.4 letters with aflibercept were comparable to those achieved with monthly ranibizumab.10 During Year 2 of the VIEW trials, patients receiving monthly PRN injections with a 12-week cap lost only -0.6 to -0.8 letters (mean number of injections: aflibercept, 4.2; ranibizumab, 4.7).31
The 2-year PrONTO trial originally validated the monthly PRN strategy and remains the benchmark for individualised therapy trials.32 Patients treated with ranibizumab improved by a mean of +9.3 letters at 12 months and required an average of 9.9 injections through 24 months. Re-injection criteria were strict and patients were followed very closely with high compliance rates. Unfortunately, subsequent PRN trials have been unable to match these excellent results. Individualised therapy for most patients with AMD involves using an effective strategy that extends the treatment interval, but some eyes fail to respond well to monthly therapy. Appropriate anatomic responses can be seen 1–2 weeks after an injection and many of these eyes can be successfully managed with 2–3-week treatment intervals, extended later to monthly or longer.33
DIABETIC MACULAR OEDEMA
Individualised therapy for AMD involves selecting an anti-VEGF drug and determining an appropriate injection frequency for each patient, but individualised therapy for patients with DR (diabetic macular oedema [DMO] patients) is considerably more complicated. Physicians must also consider alternate drug classes (such as corticosteroids), laser photocoagulation, and vitrectomy surgery.
Several lines of evidence validated the efficacy of anti-VEGF therapy for the treatment of DMO. The Diabetic Retinopathy Clinical Research Network (DRCRnet) Protocol I compared ranibizumab together with prompt or deferred (6 months) laser to intravitreal triamcinolone/laser and to laser monotherapy.34 During the first year, patients received 4-week injections of ranibizumab, followed by 2 more for persistent oedema, and up to 7 monthly PRN if retreatment criteria were met (4:2:7 strategy). During Years 2 and 3, patients were evaluated monthly or bimonthly and treated PRN. After 12 months of intensive therapy, the treatment burden during Years 2 and 3 decreased to 4–6 injections. Some surgeons perform macular laser photocoagulation to decrease anti-VEGF treatment burden but Protocol I found that prompt laser with ranibizumab decreased the 3-year injection burden by only 3 (compared to ranibizumab with deferred laser) but required 3 laser treatment sessions to accomplish this.
The prospective, multicentre RESTORE trial provided Level 1 evidence that both the European Medicines Agency (EMA) and UK National Institute for Health Care Excellence (NICE) used to approve ranibizumab.35 Patients were randomised to ranibizumab monotherapy (without rescue laser), ranibizumab plus laser, or laser. After 1 year, the laser arm became eligible for ranibizumab. Interesting outcomes included the following: patients receiving ranibizumab monotherapy improved by nearly 2 letters more than those receiving ranibizumab plus laser; laser patients who crossed over to ranibizumab after 12 months caught up (equal VA improvement) to the ranibizumab plus laser group by 36 months; patients with baseline central retinal thicknesses <400 mm achieved comparable VA improvements with laser as those receiving ranibizumab. Results from RESTORE and Protocol I suggest that ranibizumab monotherapy produces better visual outcomes than ranibizumab with prompt laser.34,35
The Phase II READ-2 trial showed that ranibizumab was superior to macular laser photocoagulation but eyes receiving 2-month injections appeared to be under-treated.36 The Phase III RISE and RIDE trials resulted in VA improvements of +8.5 to +9.9 letters with monthly ranibizumab injections over 3 years.7 Similar improvements were seen in the 0.3 mg and 0.5 mg arms but as there appeared to be a dose-dependent incidence of stroke, the 0.3 mg dose received FDA approval. Patients who were originally randomised to laser crossed over to 0.5 mg ranibizumab after 24 months, and experienced improved VA over 12 months, but failed to catch up to the ranibizumab groups. During a 2-year extension, patients received an average of 3.8 injections per year and VA remained stable.37
Aflibercept was shown to be superior to laser in the Phase III VIVID and VISTA registration trials.11 Monthly and bimonthly (after 5 monthly injections) injections produced VA improvements of +10.5 to +12.5 letters at 12 months. These trials produced the first evidence that bimonthly treatment intervals (with aflibercept) could produce outcomes similar to those with monthly therapy.
The DRCRnet Protocol T trial was the first head-to-head comparison of aflibercept, bevacizumab, and ranibizumab for the treatment of DMO. Patients were injected monthly until dry or stable and then monthly PRN through 12 months.38 Starting at 6 months, rescue laser was permitted for persistent oedema. Based on a pre-planned sub-analysis, patients with baseline VA of 20/40 or better improved equally (+8 letters) with each drug; however, patients with baseline VA of 20/50 or worse improved more with aflibercept (+18.9 letters) than with either bevacizumab (+11.8) or ranibizumab (+14.2). Patients with VA of 20/50 or worse required fewer aflibercept injections (10) and lasers (37%) than patients receiving bevacizumab (11, 65%) or ranibizumab (11, 50%).
The RETAIN trial produced the best evidence supporting the use of a T&E strategy for DMO.39 This 24-month, single-masked, prospective trial compared T&E plus laser, T&E, and PRN ranibizumab regimens in patients with DMO. The VA improvements in patients receiving T&E plus laser, T&E, and PRN were similar (+5.9, +6.1, and +6.2 letters, respectively) as were the mean numbers of injections (12.4, 12.8, and 10.7, respectively) but patients treated with T&E required 46% fewer clinic visits.
Two sustained release steroid inserts have been approved for the treatment of DMO. The 3-year Phase III MEAD trials randomised patients to receive the dexamethasone delivery system (DDS, Ozurdex®, Allergan, California, USA) or sham injections.40 More patients receiving the insert improved by at least +15 letters (22.3%) compared to sham (12.0%). Patients were eligible to receive the DDS every 6 months, though most clinical trials suggest that its duration of action is only 3 months. This may have resulted in undertreatment of patients receiving the DDS. The 3-year Phase III FAME trials randomised patients to receive the 36-month fluocinolone acetonide insert (Iluvien®, Georgia, USA) or sham.41 More patients receiving the 0.19 mg insert (28.7%) improved by at least 15 letters compared to sham (16.2%). In a post hoc subset analysis, patients with chronic (>3 years duration) oedema improved more than those with non-chronic oedema.42
Because permanent loss of vision is slower to develop in patients with DMO than in those with AMD, the risks associated with early undertreatment of DMO can usually be managed later by aggressive treatment when needed. This has reassured surgeons and encouraged the use of individualised therapy for DMO. Anti-VEGF injections are generally used as first-line therapy, though intravitreal corticosteroids may be used in patients who want fewer clinic visits and do not have steroid-responsive intraocular pressure elevations. Patients with DMO for at least 3 years may benefit more from corticosteroids than anti-VEGF drugs.
Protocol T data suggest that aflibercept may be the drug of choice for patients with moderate-to-severe vision loss due to DMO because of its greater durability and superior ability to resolve macular oedema. Some surgeons question the applicability of Protocol T recommendations to clinical practice because of the complex re-treatment guidelines and the labour-intensive Early Treatment Diabetic Retinopathy Study (ETDRS) refractions and VA measurements. Nonetheless, many physicians now choose their anti-VEGF drug based on the 20/50 VA cut-off from Protocol T. The pivotal anti-VEGF trials required that eyes responding poorly to injections receive laser photocoagulation, but physicians in clinical practice will frequently switch to a higher VEGF binding affinity drug or a sustained release corticosteroid (Figure 2).
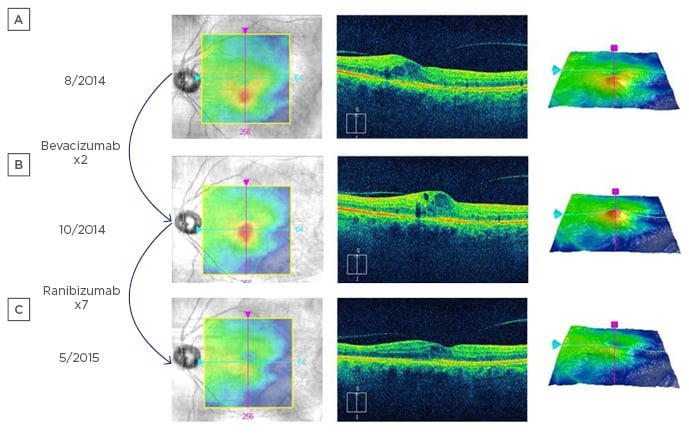
Figure 2: An eye with diabetic macular oedema.
A) An eye with diabetic macular oedema at baseline; B) two injections of bevacizumab were performed without improved oedema; C) after switching to ranibizumab (a drug with higher vascular endothelial growth factor binding affinity), the oedema resolved and visual acuity improved.
Direct comparisons of monthly to PRN and T&E strategies have not been adequately studied but inter-trial comparisons suggest that individualised strategies may produce 1–2 letters less improvement. DA VINCI showed that PRN aflibercept injections improved VA comparably to monthly and bimonthly injections (+12.0 versus +13.1 versus +9.0 letters) through 52 weeks.43
Pregnant patients with DMO present a unique treatment challenge. Anti-VEGF therapy may be relatively contraindicated in patients with several thromboembolic conditions, but pregnancy may be the only true absolute contraindication to intraocular anti-VEGF injections because of the risk of spontaneous abortion. Pregnant patients with DMO should be considered for either intraocular drug delivery system or observation with anti-VEGF treatment after delivery. Delaying therapy until after delivery should be carefully discussed with patients since 1–2 years of monthly therapy may be required before the vision catches up to where it would have been with prompt therapy.
The DRCRnet Protocol S trial demonstrated that 10 injections of ranibizumab were as effective as pan-retinal photocoagulation for the treatment of proliferative DR over 2 years.44 Differences in mean VA (+2.2 letters in favour of ranibizumab) were not statistically significant but mean area under the curve measurements favoured ranibizumab (+4.2 letters, p<0.001). Ranibizumab-treated eyes had better preservation of visual fields and developed fewer complications of proliferative DR (p<0.001 for both).
RETINAL VEIN OCCLUSIONS
Ranibizumab and aflibercept have been approved for the treatment of macular oedema due to RVO based on the results of pivotal branch (BRAVO, VIBRANT)9,14 and central (CRUISE, GALLILEO, and COPERNICUS)8,12,13 RVO trials. Six monthly injections produced mean VA gains from +14.9 to +18.0 letters. After the 6-month primary endpoint in each study patients were injected monthly PRN, with laser and sham injection patients eligible to cross-over to anti-VEGF therapy. Patients in each laser/sham injection arm experienced improved VA after receiving anti-VEGF therapy, but by 12 months none approached the visual acuities of those in the anti-VEGF arms. From 12–24 months, patients were assessed and treated every 3 months in HORIZON (the BRAVO and CRUISE extension);45 those in the ranibizumab arms lost -1.7 letters (BRVO) and -4.2 letters (CRVO). By 24 months, patients in the laser arm of BRAVO who were crossed over to ranibizumab nearly caught up to those in the 0.5 mg ranibizumab arm.
Patients receiving aflibercept in the CRVO trials lost 1–3 letters of VA after switching to bimonthly PRN therapy between Weeks 24 and 48, and an additional 2–3 letters at Weeks 48 and 96 (or 76) during which time they were assessed bimonthly.46,47
The DDS has been approved for the treatment of RVOs based on the results of the GENEVA trials.48 Patients receiving single injections achieved VA improvements of +8 to +10 letters at 3 months with a gradual decrease toward baseline by 6 months. Dexamethasone inserts appear to be more effective than bevacizumab at decreasing oedema but have not been shown to produce superior VA improvements.49 The DDS is being used as salvage treatment in patients who fail primary anti-VEGF therapy in the ongoing SCORE 2 trial.50
The pivotal RVO trials consistently show progressive loss of vision over time. As patients lose VA when injections are given PRN and treatment intervals are lengthened, their central retinal thickness slowly increases. This leads us to believe that undertreatment plays a role in deteriorating visual function. Multicentre prospective PRN and T&E trials have not been performed on patients with vein occlusions and until such data become available, physicians may wish to treat patients with RVO regularly for extended periods of time and resist the temptation to decrease the intensity of therapy.
SUMMARY
Individualised therapy for chorioretinal vascular diseases produces good results but mean visual acuities generally lag 1–3 letters behind those achieved with monthly therapy during the first year. Long-term, prospective data from multicentre controlled trials are not available, but AMD and RVO studies suggest that this gap widens with time. PRN treatment protocols decrease the number of injections and drug costs but frequent visits to physicians’ offices do not change examination and imaging costs. The indirect costs (travel, lost productivity, costs incurred by accompanying individuals) of PRN therapy remain high.
T&E regimens decrease the number of injections compared to monthly therapy, but not compared to PRN. Since office visits may decrease by 40%, both direct and indirect costs will decrease. Compared to monthly and PRN regimens, T&E regimens decrease the costs of clinic visits and ancillary testing. More injections are needed during the first year with T&E than with PRN regimens. Since some national healthcare systems do not cover drug costs, T&E regimens may not be practical in some countries. Short-term VA results with T&E appear good but head-to-head trials with monthly therapy have not been performed. Use of T&E is supported in patients with AMD and should be safe and effective in patients with DMO where physicians can revert to monthly therapy in a timely manner if needed. Safe and effective use of T&E in central RVO patients has not been demonstrated. Further investigations into individualised therapy are needed to promote compliance, and decrease treatment costs and complications, while maintaining high levels of effectiveness.








