Meeting Summary
This symposium took place at the 58th Annual Meeting of the European Association for the Study of Diabetes (EASD) in Stockholm, Sweden. The first speaker was John L. Sievenpiper, who discussed the pathophysiology of postprandial hyperglycaemia and how it may impact the risk of cardiovascular disease (CVD), peripheral vascular disease, insulin resistance, and other comorbidities in patients with Type 2 diabetes (T2D). Sievenpiper then reviewed various pharmacological interventions that target postprandial glucose (PPG) and insulin levels, including incretin therapies and α-glucosidase inhibitors, such as acarbose. Data presented showed that a low glycaemic index (GI) diet can improve glycaemic control and reduce cardiometabolic risk factors in patients with Type 1 diabetes (T1D) and T2D. Sievenpiper then presented data on novel non-pharmacological approaches that target PPG, including mulberry leaf (Morus alba L) extract (MLE), which has α-glucosidase inhibitor activity, reducing PPG and insulin responses to sucrose. The second speaker, Bo Ahrén, presented data on the effects of whey protein (WP) and branched-chain amino acids (BCAA) on PPG management and as a potential intervention for postprandial hyperglycaemia. They also discussed the mechanisms underlying the effects of WP, and highlighted data presented at the 2022 58th Annual EASD meeting by Johansen and colleagues on a novel micelle microgel technology. WP microgels (WPM) deliver highly concentrated and lower calorie doses of WP, with the potential to be developed clinically as therapeutics for T2D. The symposium concluded with a question and answer session between panel members and the audience. Ian J. Neeland was the meeting moderator.
Welcome and Introduction to the First Speaker
Ian J. Neeland
Neeland welcomed the delegates to the industry symposium and introduced the first speaker, Sievenpiper. Sievenpiper has established an internationally recognised research programme focused on using a combination of randomised controlled trials (RCT) and epidemiological approaches to address essential questions relating to clinical and public health measures regarding diet and the prevention of cardiometabolic disease, with a particular interest in the role of sugars, the quality of dietary carbohydrates, and therapeutic plant-based diets. Sievenpiper has a direct role in knowledge translation, with appointments to nutrition guidelines committees as part of Diabetes Canada,the EASD, Canadian Cardiovascular Society (CCS), and Obesity Canada.
Implications of Elevated Postprandial Glucose
John L. Sievenpiper
Diabetes is a global epidemic. In 2021, more than 500 million people worldwide were living with diabetes, and this is projected to increase by 46% to 784 million by the year 2045.1 Heart disease driven by the dual epidemics of obesity and diabetes remains the leading cause of death worldwide.2
Diagnosis of Type 2 Diabetes
The criteria for diagnosing T2D include a fasting plasma glucose (FPG) ≥7.0 mmol/L, a 75 g oral glucose tolerance test (2-hour plasma glucose) ≥11.1 mmol/L, a glycated haemoglobin (HbA1c) ≥6.5%, and random plasma glucose ≥11.1 mmol/L.3-6 The known clinical effects of T2D are the basis for clinical follow-up evaluation and include tests for diabetic nephropathy, neuropathy, and retinopathy.7-9
Postprandial Glucose is the Gold Standard for Assessing Diabetes
Postprandial hyperglycaemia is one of the earliest manifestations in response to insulin resistance and insulin secretory dysfunction in the pathogenesis of T2D. Dysglycaemia presents earlier with PPG versus FPG.10 In particular, β-cell dysfunction, which represents insufficient insulin secretion at a given level of insulin resistance manifesting as high glucose, is seen earlier with PPG than with FPG.11
The DETECT-2 Collaboration, which pooled nine studies of more than 44,000 individuals from five countries, showed that the 75 g oral glucose tolerance test is more sensitive and detects diabetes more effectively than with FPG or HbA1c, based on retinopathy.12 The DECODE study included more than 29,000 individuals over 11 years of age, and found that the 2-hour PPG was associated with increased CVD mortality even within a normoglycaemic range.13 The DECODE study also demonstrated that at any level of FPG, an elevated PPG is associated with increased CVD mortality.14,15
Pharmacological Interventions That Target Postprandial Glucose
There are several pharmacological interventions to target PPG, including incretin therapies, mealtime insulin, and α-glucosidase inhibitors, such as acarbose. Sievenpiper’s colleague, David J.A. Jenkins, studied acarbose before the GI was recognised as a concept. In a randomised crossover study in 1981, four healthy individuals with a normal glucose tolerance were given a low dose of acarbose of 50 mg three times a day (TID) with meals and 25 mg TID with snacks. The study demonstrated that acarbose reduced PPG over 24 hours compared with placebo.16
The STOP-NIDDM trial randomised 1,429 individuals with impaired glucose tolerance (IGT) to high-dose acarbose (100 mg TID), and followed them for 3.3 years.17,18 There was a 25% risk reduction in T2D with acarbose compared with placebo. In a prespecified secondary analysis of the trial, there was a 49% risk reduction in cardiovascular events with acarbose versus placebo.19 A meta-analysis of seven studies powered to examine cardiovascular outcomes included 2,180 patients with T2D and found that acarbose was associated with reductions in myocardial infarction and total cardiovascular events in T2D.20
The Acarbose Cardiovascular Evaluation (ACE) trial, designed and powered to investigate coronary events, enrolled 6,522 Chinese patients with IGT and established coronary heart disease and found that low-dose acarbose (50 mg TID) reduced the risk of T2D but not cardiovascular events over a median 5-year follow-up.21,22 The reduction in T2D confirmed the findings of STOP-NIDDM, but whether these findings translate to a real-world reduction in cardiovascular events downstream remains questionable.
Are There Guidelines-Based Nutritional Analogies of Acarbose?
The GI was first introduced by Jenkins et al.23 in the early 1980s as a methodology to rank and classify carbohydrate foods as low (≤55), medium (56–69), and high (≥70) GI, based on their ability to raise blood glucose levels with glucose as the reference, which has a GI of 100.23 The GI has since been incorporated into clinical practice guidelines from organisations such as the EASD and Diabetes Canada. Low GI foods are absorbed over the length of the small intestine, whereas high GI foods are absorbed proximally and at a faster rate, resulting in a significant and rapid rise in PPG levels and glucose variability. Major diabetes and CVD clinical practice guidelines worldwide recommend low GI/GL diets as an effective strategy for glucose management.24
Studies of low GI foods and acarbose indicate that acarbose can influence the GI of a meal, thereby possibly lowering the overall glycaemic load (GL). In 1979, a randomised study in eight individuals conducted by Jenkins et al.25 compared placebo with guar gum (14 g) alone, acarbose (50 mg) alone, and guar gum (14 g) plus acarbose (50 mg).25 Guar gum and acarbose produced similar 2-hour PPG reductions, with additive suppression in PPG when the two compounds were administered together.25 The findings from a systematic review of RCTs and meta-analysis study, conducted by Jovanovski et al.,26 supported that viscous fibre supplements provided glycaemic control beyond usual care and should be considered in the management of patients with T2D.26 An average dose of approximately 13 g of viscous soluble fibre (β-glucan, guar, konjac mannan, psyllium) during 8 weeks led to a clinically meaningful 0.61% reduction in HbA1c.26 These findings would meet U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) criteria for new drug development.
In 1980, further studies by Jenkins et al.27 showed that legumes slowed carbohydrate digestion during a 3-hour period, which was reflected in a lower rise in PPG.28 Moreover, a meta-analysis by Sievenpiper et al., which included 41 RCTs with 1,674 individuals, with and without T2D, found that pulses consumed as part of a low GI and high fibre diet reduced HbA1c by 0.48% in patients with T2D. In addition to improving glycaemic control, low GI (≤55) diets have been shown to improve cardiometabolic risk factors in individuals with both T1D and T2D.30 The systematic review and meta-analysis commissioned by the Diabetes and Nutrition Study Group to update the guidelines found meaningful reductions of 0.3% for HbA1c, a modest but clinically significant difference of 0.4 mmol/L for FPG, and approximately a 5% decrease in lipids.
In contrast, high GI/GL diets were associated with an increased incidence of diabetes in a meta-analysis of 24 prospective cohort studies with a 4-year to 22-year follow-up.31 For GI, the combined relative risk (RR) was 1.27 (range: 1.15–1.40) per 10 unit increase and 1.87 (range: 1.56–2.25) across the global range, while for GL the combined RR was 1.26 (range: 1.15–1.37) per 80 g/day and 1.89 (range: 1.66–2.16) across the global range.31 High GI/GL diets have also been associated with an increased incidence of coronary heart disease.32 In a meta-analysis of 11 prospective cohort studies, including 350,000 individuals and 10,400 events over 11.4 years of follow-up, the overall RR for GI was 1.24 (range: 1.12–1.38) per 10 unit increase and 2.71 (range: 1.47–4.40) across the global range.32 The RRs for GL were 1.44 (range: 1.25–1.65) per 65 g/day and 5.5 (range: 3.1–9.8) across the global range.32
Supplements That Target Postprandial Glucose
The MLE (Morus alba L) has been demonstrated to have α-glucosidase inhibitor activity and was investigated in a double-blind RCT of 38 healthy individuals.33 Subjects were randomised to consume either 250 mg MLE, containing 12.5 mg of the active component deoxynojirimycin, or placebo and followed for 2 hours.33 MLE was found to reduce PPG and insulin responses following administration of 75 g sucrose.33
In a crossover study that included 30 patients with T2D found that the same MLE combined with 1.75 g fibre, 0.75 μg vitamin D3, and 75 μg chromium reduced 3-hour PPG and insulin responses to a mixed meal tolerance test (55.4 g of carbohydrate Figure 1).34
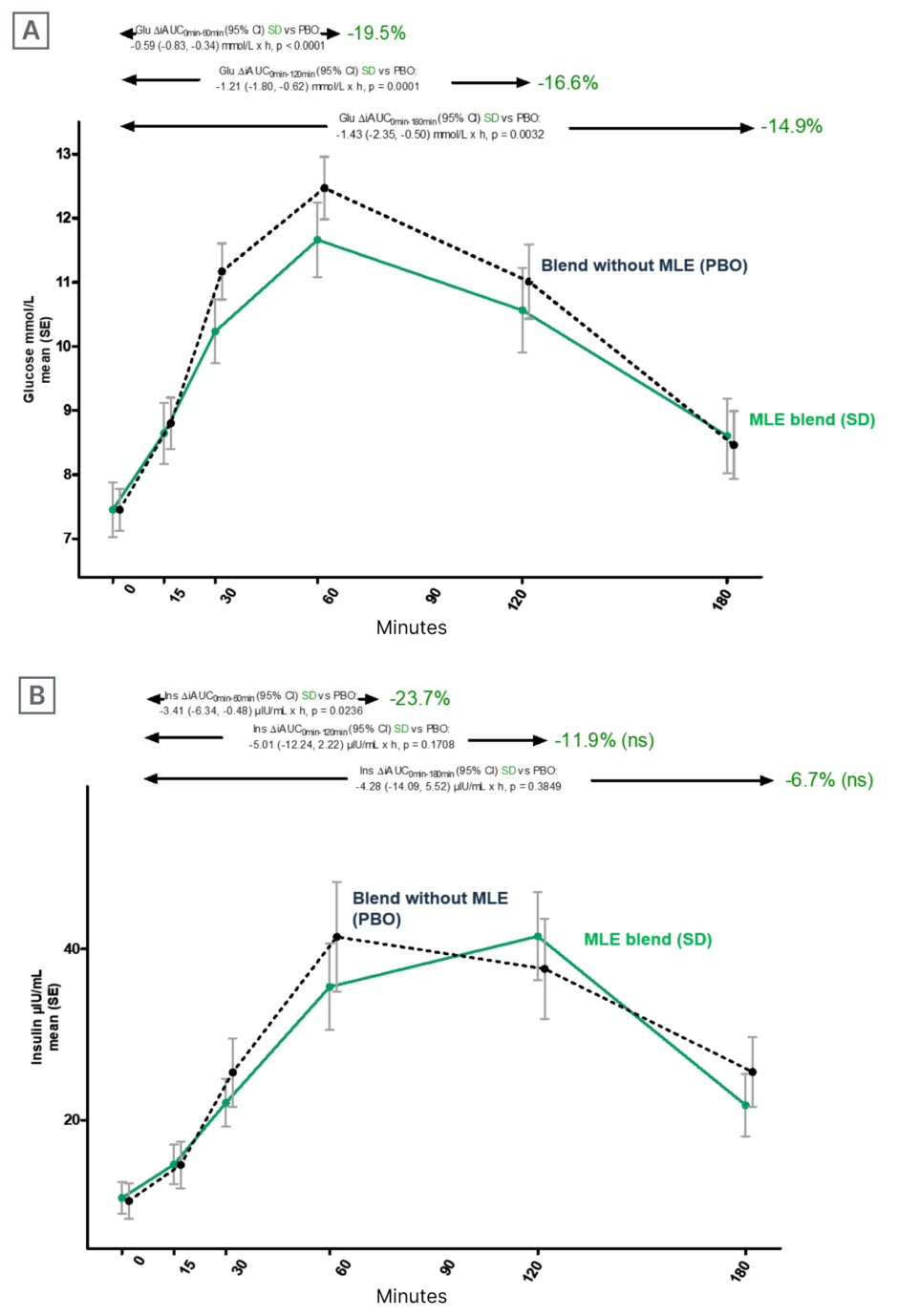
Figure 1: Effects of mulberry leaf extract on glucose (A) and insulin trajectories (B).
AUC: area under the curve; CI: confidence interval; Glu: glucose; Ins: insulin; MLE: mulberry leaf extract; ns: not significant; PBO: placebo; SD: standard deviation; SE: standard error; vs: versus. Adapted from Mohamed et al.34
In adults with prediabetes, significant results were also found with MLE (Morus alba L) when 38 individuals with IGT in a double-blind, parallel RCT were randomised to receive either 5 g/day (18 mg deoxynojirimycin) or placebo with a 4-week follow-up and showed MLE reduced PPG and insulin responses to a mixed meal tolerance test.35,36 Similar findings of α-glucosidase inhibition have been reported with another MLE product (Morus nigra) in patients with T2D.37 In a controlled clinical study of 100 patients with T2D, 50 patients were treated with the MLE extract for 3 months, and 50 patients were treated with placebo.37 At 3 months, fasting blood glucose and HbA1c were significantly reduced in the MLE-treated patient group.37
Conclusion
The epidemic of T2D and its downstream cardiometabolic complications threaten healthcare systems worldwide. PPG is the gold standard for diagnosing and probing the natural history of T2D. Pharmacological interventions that aim to lower PPG, such as α-glucosidase inhibitors, improve glycaemic control and cardiometabolic risk factors, and reduce incident diabetes. Guideline-based nutritional interventions aim to lower PPG (e.g., low GI/GL, viscous fibre, and pulses). These recommendations represent a nutritional analogy of acarbose, showing similar improvements in glycaemic control, cardiometabolic risk factors, and the reduction of diabetes and complications such as CVD. Supplements that target PPG, for example MLE, may share similar advantages; however, further studies and more data are needed.
Introduction to the Second Speaker
Ian J. Neeland
Neeland introduced the second speaker, Ahrén, who has been directly involved in the development of new targets and compounds for the treatment of T2D. Ahrén has a special interest in the development of new treatments based on the incretin hormone glucagon-like peptide 1 (GLP-1), and its inhibitory enzyme dipeptidyl peptidase 4 (DPP-4). Ahrén has published several original and review articles in the area of islet cell function with the special aim of understanding the regulation and mechanisms of normal pancreatic islet function, and the mechanisms and consequences of islet cell dysfunction as a key factor underlying T2D.
Nutritional Approaches for Postprandial Glucose Management Focus on Whey Protein
Bo Ahrén
Milk has 3.5 g of protein per 100 ml, which constitutes 20% of the energy provided by milk. There are two types of protein in milk: WP (approximately equal to 20% of the protein in cow’s milk and 60% in human milk) and casein protein (approximately equal to 80% of the protein in cow’s milk and 40% in human milk).
Constituents of Whey Protein
WP is the protein in the liquid remaining after milk has been coagulated during cheese production. This protein fraction is acid soluble and rapidly delivered to the gut after ingestion. WP consists of a mixture of proteins, the majority being β-lactoglobulin (approximately 50–60%), and the remaining including α-lactalbumin (approximately 10–20%), glycomacropeptide (12–20%), albumin (approximately 5–10%), and Igs (approximately 5–10%). WP contains a large fraction of the BCAAs leucine, isoleucine, and valine, which have been shown to play an important role in protein synthesis and other metabolic actions.38 Today, WP is readily available as a supplement for muscle growth and development, but it also has the potential to treat PPG.
Branched-Chain Amino Acids Are Important for the Metabolic Action of Whey Protein
WP increases circulating levels of BCAA in T2D. This was demonstrated in an RCT by King et al.,39 whereby 11 subjects with T2D were randomised to receive 15 g intact or hydrolysed WP before a breakfast meal.39,40 Plasma BCAA concentrations were measured after breakfast alone, and prior to consumption of 15 g intact and hydrolysed WP before breakfast. After breakfast, there was no change in plasma concentrations of valine, leucine, and isoleucine, while concentrations more than doubled in 20–40 minutes when WP was consumed immediately before breakfast.39
The increased BCAA concentration following WP consumption is important because BCAAs are potent stimulators of insulin secretion. BCAAs have been extensively studied in isolated islets and animal studies. In 2008, Kalogeropoulou et al.41 compared the insulin response in 13 healthy subjects after receiving 2 g leucine with 25 g glucose versus 25 g glucose alone.41 Leucine resulted in a doubling of the insulin concentration after glucose compared to glucose alone, which was associated with a reduction in glucose levels.
WP stimulates insulin secretion through three mechanisms. One, BCAAs directly stimulate islet β-cells to secrete insulin, and the other two effects are indirect, whereby WP bioactive peptides stimulate the secretion of the incretin hormones, gastric inhibitory polypeptide (GIP) and GLP-1.42,43 Furthermore, WP inhibits the action of DPP-4, the enzyme responsible for GLP-1 and GIP inhibition, which leads to a sustained active concentration of GLP-1 after meal ingestion.42
Effects of Whey Protein on Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide, and Dipeptidyl Peptidase-4
In 2009, Ma et al.44 conducted the first human study showing a marked increase in GLP-1 and GIP levels following WP consumption.44 Eight subjects with T2D consumed 55 g WP either together with a breakfast meal or as a 30-minute preload in which GLP-1 and GIP concentrations were measured. Concentrations of GIP and GLP-1 increased after breakfast alone, but there was a potentiation of the response after WP was given with the test meal, concluding that WP was a particularly potent stimulator of GLP-1 when given as a preload.
The inhibition of DPP-4 by WP was shown in 2006 by Gunnarsson et al.42 in Ahrén’s group. Mice were given 75 mg glucose alone or together with 75 mg WP. The WP and glucose treatment led to a significant potentiation of insulin levels and a reduction in glucose. Both GLP-1 and GIP were increased 15 minutes after after the WP and glucose treatment. Finally, the addition of WP to glucose led to a reduction in DPP-4 activity.
Whey Protein Delays Gastric Emptying and Reduces Postprandial Glucose
WP also delays gastric emptying by inhibiting muscle cell activity and through the release of GLP-1. In Ma et al.’s44 2009 study, discussed above, subjects consumed a radio-labelled mixed meal.44 Scintigraphy was used to measure the amount of food remaining in the stomach after specific time periods.44 Sixty minutes after subjects consumed the control mixed meal (no WP), 25% of the meal remained in the stomach.44 When WP was given as a preload, 70% of the mixed meal remained in the stomach after 60 minutes.44
WP reduces PPG via several mechanisms.42,43 It increases insulin secretion, which causes a reduction in hepatic glucose production and an increase in peripheral glucose utilisation, and it delays gastric emptying, which causes a delay in glucose absorption.
Effects of Whey Protein in Healthy Subjects and Patients with Type 2 Diabetes
Gunnerud et al.45 conducted a dose–response study of WP in 12 healthy subjects who received 4.5, 9, and 18 g WP together with 25 g glucose.45 WP reduced PPG and increased insulin and BCAA levels in a dose-dependent manner.45 Ahrén conducted a randomised clinical trial with Jakubowicz and colleagues in Israel, in which 15 subjects with T2D were given 50 g of WP 30 minutes before a mixed breakfast meal.46,47 Results showed a significant reduction in PPG, the incremental area under the curve (AUC) was reduced by 25%, and peak glucose levels were reduced from 16 mmol/L to 10–11 mmol/L.46 Also, the AUC for insulin doubled and GLP-1 concentrations more than doubled.46
Several studies have been conducted on the effects of WP in T2D. The greatest efficacy has been observed when WP is taken 30-minute prior to a meal and at high doses (50–55 g), which while efficacious, contributes an extra 200–220 kcal.44,46
In 2017, the long-term effects of WP were investigated by Ahrén together with Jakubowicz.48 A total of 19 subjects with T2D were given 28 g WP as a preload 15 minutes before a standardised breakfast every morning for 12 weeks.48 The results showed a persistent glucose reduction and increase in insulin and GLP-1 levels.48 In addition, WP resulted in a reduction in both HbA1c and body weight.48
The reduction in body weight observed with WP consumption has been thought to be due to the bioactive peptides and BCAAs activating the satiety centre in the hypothalamus, resulting in an acute enhanced feeling of fullness and suppressed appetite, which over the long-term, has been associated with a reduction in body weight.49 WP has also been shown to stimulate the release of gastrointestinal hormones involved in the regulation of appetite and satiety, including GLP-1, peptide YY (PYY), cholecystokinin, and ghrelin.42,43,49,50
Limitations of Traditional Whey Protein Formulas to Regulate Postprandial Glucose
Translating the benefits of WP to clinical use is challenging for two main reasons. One, the greatest efficacy has been observed when WP is taken as a preload 30 minutes prior to a meal; and two, a high dose of 50–55 g is required. With this type of regimen, compliance is a challenge and creates the burden of having to plan ahead, as well as contributes an additional 200–220 kcal. Therefore, to be able to use WP as an effective intervention, there is a need for a new format or technology that enables the use of a lower, more potent dose.
Micelle Technology to Administer Whey Protein
A novel formulation of WP has been developed, which allows for a smaller, more highly concentrated formula that is more readily absorbed.51 Micelle technology generates WPM and reduces the size of WP into 250 nm micelles, producing a highly concentrated product delivered in a small volume. This highly concentrated WPM formula delivers a low dose of 10 g WP at only 40 kcal. The WPM formula acts quickly, within 10–15 minutes of consumption, and holds promise for translating the knowledge on WP into the clinic.
On behalf of Johansen et al., Neeland presented the study findings of this novel formulation as a short oral abstract presentation at the 58th EASD 2022 meeting.51 In a randomised, placebo-controlled, crossover study, 26 adults with T2D were randomised to receive the WPM containing 10 g of WP (40 kcal) versus placebo (0 kcal). It was taken as a 125 mL shot, 15 minutes prior to consumption of a 250 g pizza lunch (622 kcal). Glucose, insulin, BCAA, and gut hormones (GLP-1, GIP, and PYY) were assessed.51 The results showed that the 10 g WP premeal shot significantly reduced PPG levels, measured by iAUC.51 The iAUC was reduced by 22% during the first 2 hours and 18% over 3 hours.51 This was associated with an increase in early phase insulin secretion, which is critical for suppressing hepatic glucose production after a meal. The AUC was augmented by 61% at 1 hour and 14% at 3 hours. Regarding gut hormones, the AUC for GLP-1 increased by 66% during the first 2 hours. In contrast, there was no significant effect of WPM on PYY and GIP.51
The effects of WPM on gastric emptying were also examined using an acetaminophen (paracetamol) test.51 Paracetamol was administered together with the meal, and levels were measured as an indirect marker of gastric emptying.51 The results showed that plasma concentrations of paracetamol were reduced by 17% during the first 45 minutes compared with placebo, signalling a delay in gastric emptying with WPM administration.51 Finally, the study showed that BCAA concentrations increased dramatically after WPM administration compared with placebo.
In another study by Johansen, a 125 mL premeal WPM shot (10 g; 40 kcal) was given to 102 subjects with T2D or prediabetes prior to consumption of a lunch meal.52 Before WPM consumption and 15 minutes afterward, self-reported sensations were scored on a scale of 1–9 as hungry (1–3), satisfied (3–6), or full (7–9).52 The number of subjects reporting that they were hungry significantly decreased following WPM, and the number stating they were satisfied significantly increased.52
Conclusion
WP degrades to a mixture of bioactive peptides and BCAAs and has been shown to stimulate the secretion of insulin, GIP, GLP-1, and PYY. Through several mechanisms, studies have demonstrated that WP can effectively reduce PPG in T2D, with the greatest efficacy observed when WP is administered at high doses 30 minutes before a meal. A novel micelle technology allows for WP to be delivered as a highly concentrated formula in a smaller volume, which can be taken 15–30 minutes before a meal. WPM has been shown to reduce PPG, increase insulin, GLP-1, and BCAA levels, and increase satiety. Longer-term studies are needed to fully understand the impact and potential of this new technique.
Question and Answer Session
Ian J. Neeland, John L. Sievenpiper, and Bo Ahrén
The symposium concluded with a question and answer session among panel members and the audience. Topics included reduced body weight with WP despite increased insulin secretion, the effects of a general protein mixture versus WP, the epidemiology of WP intake and T2D prevalence, targeting low GI or low GL to lower PPG, the applicability of WP in T1D, and the mechanisms linking PPG excursion and acarbose with cardiovascular events.







