The race to advance the field of hematology has been fierce over the past few decades. Major discoveries have changed the landscape forever, but how can the industry shape up to better serve patients?
Words by Jade Williams
At the beginning of October, a pharmaceutical company took an important step forward for patients with blood disorders. In this case, for people with haemophilia.
In a first-of-its-kind global survey, Sanofi partnered with The Harris Poll to find out what life is like for patients living with the blood disorder today. The results provided new insight into the evolving needs of the haemophilia community, while unveiling unmet needs for industry to address.
While the hematology space has developed dramatically over the past few decades, the industry needs to go further than just providing new treatments. How can the head honchos in haematology ensure that this therapy area continues to thrive with patients in mind?
Rapid success
“The haematology space is a largely underappreciated area of fast development in pharma,” comments Katelyn Gilmour, Global Medical Affairs leader, Haemato-oncology, Clinical Sequencing Division, Thermo Fisher Scientific.
Not only has it grown significantly in recent decades, but it is forecasted to keep growing at pace. In 2021, the hematology market was worth a massive $2.13bn, according to Data Bridge Market Research. By 2029, it is predicted to grow by almost another billion.
What’s behind the past and predicted surges? Well, over the years, the therapy area has benefited greatly from advances in technology, with an interesting case study being the development and refinement of bone marrow transplants.
In the early 1980s, this treatment for diseases such as leukaemia was reserved for a lucky few due to the difficulty of finding donor matches. A decade later, transplants using stem cells from umbilical cord blood became available – allowing for mismatched and half-matched transplants, such as from parent to child.
In addition, the establishment of donor registries has expanded the pool of potential donors, making it easier for patients to find a match. There are now more than 40 million potential bone marrow donors worldwide.
While bone marrow transplantation is a brilliant example of how a highly specialised and life-saving haematology treatment has become more accessible, a number of treatments for blood disorders and cancer remain out of reach for many.

The ‘A’ word
As with any groundbreaking therapy, the greatest challenge often lies in ensuring equitable access for patients. This seems to especially be the case with regards to rarer diseases such as the blood disorder haemophilia, with roughly 75% of people living with the condition residing in the developing world.
While the space has indeed witnessed tremendous progress, challenges remain on making cutting-edge haematology therapies accessible to all. One of the most significant factors contributing to this challenge lies in pricing.
Innovative treatments often come with high price tags, and the industry must work with both healthcare systems and insurance providers to ensure that life-saving therapies are within reach for all patients, regardless of their economic and geographic background.
One way in which companies can strive for better access is through partnerships. Peter Ahnesorg, Global Hematology Franchise Head, Hoffman La-Roche, says that his company, for example, has made efforts to partner with charities to ensure easier access to their medicine for haemophilia A.
Speaking to GOLD in a recent Catalyst interview, he says that the company tries to do this “where possible and where it makes sense”. And he adds that Roche strives to be innovative with access in countries “where there is literally no system” for patients to be treated with life-saving therapies.
For rare diseases such as haemophilia A and B, making medicines available in this way can be crucial. One-time treatments, such as CSL Behring’s groundbreaking haemophilia B drug, can be high cost – at $3.5m per treatment, it has been called ‘the world’s most expensive drug’ – and is clearly less accessible to people in low- and middle-income countries unless deals are made.
In the press release announcing the approval of the therapy, Lutz Bonacker, Senior Vice President and General Manager of Commercial Operations Europe, CSL Behring, stressed that the company must “ensure that as many eligible patients as possible across Europe have access to this innovative treatment”, confirming that CSL will have to work hard to ensure equitable access.
Diagnostic test troubles
Aside from concerns around access, new regulation is also posing a problem to the future growth of the hematology space. In-Vitro Diagnostic Medical Devices Regulation (IVDR) is casting a cloud over the diagnostic test development space, particularly in the haemato-oncology arena.
Although the IVDR is designed to regulate the medical device market and improve public health in the long term, Gilmour suggests that some clinical laboratories are becoming reluctant to incorporate new diagnostic tests into their routine testing workflows since its implementation.
“The process is very, very complicated,” she notes. “There are not enough notary bodies, and the different requirements make it tough.” Devices for use in haematology and haemato-oncology, such as cancer tests and genetic tests, fall under Class C of the IVDR – the second highest class based on risk profile. As a result, Gilmour says the uptake and pace of new haematological device development is slowing.
This is a challenge because diagnostic tests play a huge role in hematology. In 2022, US product revenues for instruments far outstripped those for consumables, demonstrating the widespread need for these alongside traditional treatments.
The unseen burden
While the industry has its work cut out on matters of access and innovation, the job doesn’t stop there. The mental health burden of haematological conditions is a fundamental challenge in which the industry has a role to play.
In Sanofi’s haemophilia survey, they asked patients to describe how their condition affected their mental health. Out of the patients surveyed, 59% said they often felt anxious, 54% often worried about a sudden bleeding event and 51% said they often felt depressed.
The mental burden of disease can weigh heavily on patients, and this is especially true with rarer disorders such a haemophilia and many haematological cancers.
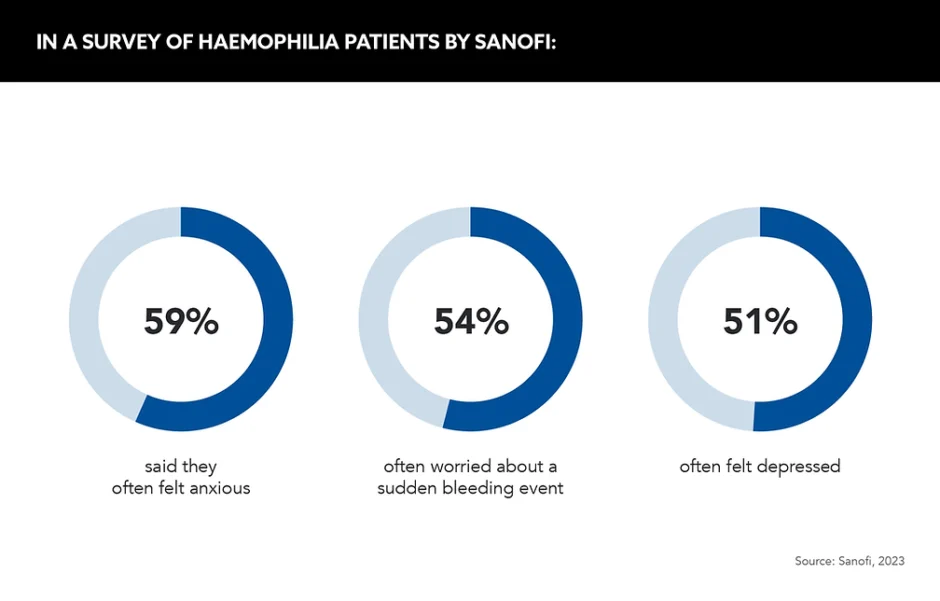
Speaking on the data’s release, Tom Snow, Global Franchise Head, Neurology and Rare Blood Disorders, Sanofi, states that “listening deeply to the community helps us provide the right support at the right time”. He adds that patients should be able “focus less on living with this condition, and more on living the lives they choose”.
The patient voice plays a monumental role in ensuring that the industry can develop treatments with the end user in mind, and this is clearly an area where the pharma industry could improve.
One way to do this, according to Gilmour, would be to reduce treatment decision wait times for patients. “One of the mental health burdens for patients can be waiting for results to come out before they get their treatment decision,” she notes. “It can sometimes be three to four weeks before the clinic makes a decision about the treatment.”
To alleviate this pressure, Gilmour suggests that healthcare and pharma teams should work together to develop integrated diagnosis reports that can be made available quickly to patients. This would help to lessen the burden and anxiety related to waiting for a decision.
Racing toward a better future
Clearly, haematology is an exciting area for access initiatives and innovation, but will this rapid growth in the sector continue in the future?
The market growth data says yes, but the reality on the ground is that progress will need to be driven by ever more innovative approaches. “The fast decision making we’ve seen in the past can lead to fast technologies that can read and sequence data much quicker,” offers Gilmour.
She is right: speed will certainly lead to better, quicker access in hematology. If the industry can continue to increase the pace of its technological advances, particularly to deliver holistic, comprehensive care, even better patient outcomes will be just around the corner.









