Pharma has danced to the beat of its own drum for decades, dominating the disco through the production of pills and injectables to solve patient health concerns. But with addiction rates for some drugs on the rise, and patients becoming more aware of their own health autonomy, should pharma be offering more to the floor?
Words by Jade Williams
Pharma has gone through exponential change in recent years. From Brexit’s arrival to COVID-19’s upheaval of the system and so much more, the industry has seen its fair share of shake-ups. One such big change has been a rise in awareness of addiction rates – stemming mostly from the opioid crisis which first reared its destructive head in the 1990s. As a result, patients are becoming far more aware of their own role in deciding which treatment plans to follow, with many choosing to partner with digital therapeutics (DTx).
DTx dynamos
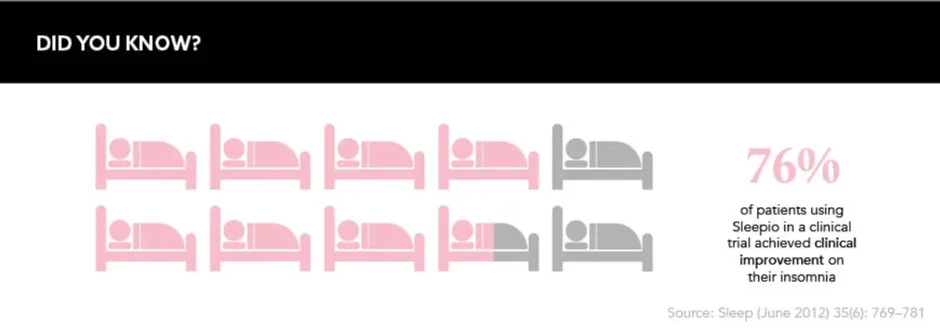
DTx danced its way into mainstream media recently after the UK’s National Institute for Health and Care Excellence (NICE)’s approval of Big Health’s digital therapeutic Sleepio, which is positioned as an alternative to sleeping pills such as zolpidem and zopiclone – the likes of which can be dependency forming. The programme uses an artificial intelligence algorithm to provide patients with cognitive behavioral therapy (CBT) for insomnia and can be offered on prescription from healthcare professionals.
NICE found that this ‘digital sleeping pill’ is not only an effective alternative to potentially addictive drugs but is also “cost saving for the NHS in primary care”, according to Jeanette Kusel, Acting Director for MedTech and Digital, NICE, speaking at the time of NICE’s recommendation. Both the public and healthcare infrastructures are clearly interested in the potential for DTx, and app-based therapies such as Sleepio offer an intriguing proposition.
Should pharma therefore be looking toward greater investment in such technologies? Quite possibly. Heather Cook, UK Director, Big Health – the company behind Sleepio – hopes that “wider adoption of Sleepio will kick start the digital therapeutics industry in the UK and across the world”. Indeed, this digital therapeutic’s approval could be the guiding hand pharma needs to shift priorities, turning away from only offering drugs and injectables towards a digital-first or hybrid approach.
The way forward is all about partnering, investing and then, ultimately, acquiring
“Leading clinical guidelines have long-stated CBT is the first-line recommended treatment for the most common mental health conditions,” states Cook, adding that CBT should be offered before or alongside medications as standard. While medications can serve as a short-term treatment for mental health concerns, not every patient will want to follow this route, nor will every HCP want to risk prescribing these often addictive substances. Put simply, this leaves ample room for DTx products to take to the floor.
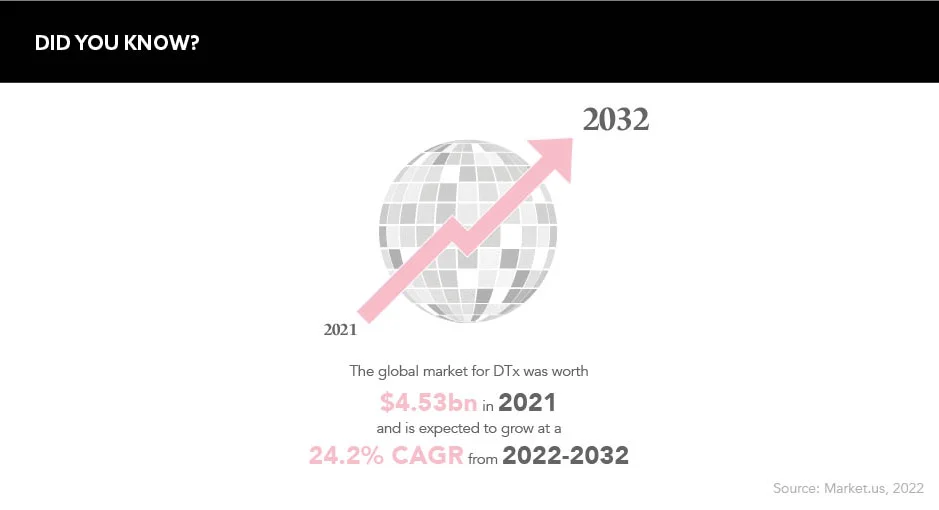
There is evidently a realm of opportunity for pharma in the DTx field. The global market for this sector of care was worth $4.53m in 2021 and is expected to grow at a 24.2% compound annual growth rate over the next 10 years. Patient expectations are changing, and the industry needs to keep in time as best as it can. Considering the potential wealth lying within this sector, and its cost-effectiveness with regards to public health systems, pharma may be foolish to let this opportunity slip through its fingers.
It takes two to tango
But while pharma could seek to develop its own DTx technologies, Eugene Borukhovich, Co-Founder and COO, YourCoach.Health, recommends that “everyone just needs to do what they do best”. Pharma already dominates the drug arena, so trying to quick-step from medicine to non-drug treatment might prove a tricky task to perform solo. Instead, Borukhovich suggests “the way forward is all about partnering, investing and then, ultimately, acquiring if there is traction in the market”.
CBT is the first-line recommended treatment for the most common mental health conditions
It is hoped within healthcare circles that patients will gain access to a wider range of choice in their treatment options. While Borukhovich doesn’t see standalone DTx products becoming a serious consideration for pharma for years to come due to the higher profit margins in speciality molecules, he predicts that these technologies could gain success in the neurodegenerative space. One such example would be Akili Interactive’s success with EndeavorRx – a prescription treatment for ADHD delivered through a video game.
DTx is taking its first tentative steps onto the pharmaceutical dance floor. Shouldn’t the industry be ready and waiting to take its hand? Cook is steadfast in her view on this, stating that “with the increasing prevalence of mental health conditions and demand on existing resources, it is imperative to increase patient choice and access to evidence-based treatment options”.
Non-drug treatments may be the solution to meeting patients’ newly-developing needs in the technological era, and pharma needs to look towards the potential that this digital field holds if it’s going to continue to lift the treatment glitter ball trophy.