BACKGROUND
COVID-19 was first identified in Wuhan, Hubei Province, China, in late 2019 and led to a worldwide pandemic, with limited understanding of how the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus caused disease. COVID-19 pneumonitis was the most commonly identified presentation but, over the course of 2020, the haematological effects of the SARS-CoV-2 virus became more evident, leading to improved protocols for anti-coagulation prophylaxis and a lower threshold for considering CT angiograms to investigate persistently unwell or deteriorating patients with COVID-19. COVID-19 is an emerging health emergency, with a lack of knowledge and literature compared to other common respiratory viral infections. The sharing of clinical experience in the medical literature, to demonstrate key complications in COVID-19 and to aid in the treatment of COVID-19, is vital to improve survival rates. Thromboembolism is a complication of COVID-19 that all healthcare individuals must be aware of; there must be a low threshold of suspicion for thromboembolism in COVID-19 and treat accordingly. It is important to consider the future management of patients with COVID-19, including whether thrombolytic therapy or enhanced prophylaxis are indicated.
INITIAL PRESENTATION
A fit and active 77-year-old male was admitted with a 5-day history of fever, productive cough, and hypoxia during the winter peak of the COVID-19 pandemic. Despite an initial negative screening test, clinical presentation, and chest radiograph findings warranted a repeat PCR test, which confirmed COVID-19 infection. Their medical history included seropositive rheumatoid arthritis treated with methotrexate, diet-controlled Type 2 diabetes (HbA1c: 65 mmol/mol [<48]), with a normal range BMI, hypertension, and hypercholesterolemia. They had never previous been in atrial fibrillation, had a cardiovascular event, pulmonary emboli, or deep vein thrombosis.
They were categorised as a moderate case of COVID-19 infection, with no evidence of pulmonary embolism on CT-arterial portography (CTPA) on Day 6 of symptom onset and a typical COVID-19 blood profile. The CTPA showed ground glass opacification, characteristic of COVID-19. Admission ferritin level was 2,500 μg/L (20–300) and his D-dimer was 2,700 ng/mL (<500). C-reactive protein was raised at 300 mg/L (0–5). White cell count (WCC) was at 6.0×109 /L (4.2–10.6), with a lymphopaenia (0.3×109 /L [1.1–3.6]). Procalcitonin was 0.33 μg/L (values <0.5 practically excludes infection). Activated partial thromboplastin time was 29 secs (25–35). Platelets were 236×109 /L (130–370) and remained within normal range throughout admission (ruling out heparin induced thrombocytopaenia).
The hospital policy included starting dexamethasone for patients with symptoms, which started less than 10 days previously and who are saturating at <90%; remdesivir in severe cases before Day 10 of symptoms; enhanced thromboembolism therapy for all (based off weight and platelet level); and antibiotic therapy (amoxicillin 2 g intravenous three times daily and doxycycline 200 mg orally once daily) is not indicted unless there was a superimposed bacterial infection or for patients with sepsis.
Dexamethasone was commenced. Their oxygen requirements increased, and they subsequently progressed to a severe infection (meeting the criteria for acute respiratory distress syndrome) with non-invasive ventilation commenced on Day 10 of symptoms (when the patient was outside the window for remdesivir). Serial procalcitonin levels were all <0.5 μg/L. Subsequently, oxygen requirements were weaned and by Day 15 they no longer required oxygen supplementation.
CASE PRESENTATION
Abdominal Pain: Renal and Splenic Infarcts
On Day 16 of symptoms, the patient developed moderate right upper quadrant abdominal pain and had evidence of a neutrophilia (WCC: 20.9×109 /L, with neutrophils: 20.2×109 /L [2.0–7.1]), plus an increasing C-reactive protein of 157 mg/L and a cholestatic picture, with a raised alkaline phosphatase of 170 U/L (30–130) and an alanine aminotransferase of 64 U/L (0–45). An ultrasound of the liver showed a fatty liver but was otherwise normal.
On Day 18 of symptoms the patient reported ongoing, now generalised, abdominal pain and had evidence of abdominal distension with reduced bowel sounds on examination. Inflammatory markers and neutrophil counts increased, and with a lactate at the upper limit of normal (2 mmol/L [0.5–2.0]), an abdomen CT was performed. This demonstrated new multiple splenic infarcts (Figure 1), which were not seen on admission CTPA and a probable wedge infarct within the interpolar segment of the right kidney (Figure 2). Parts of the caecum were distended with mild inflammatory changes seen.
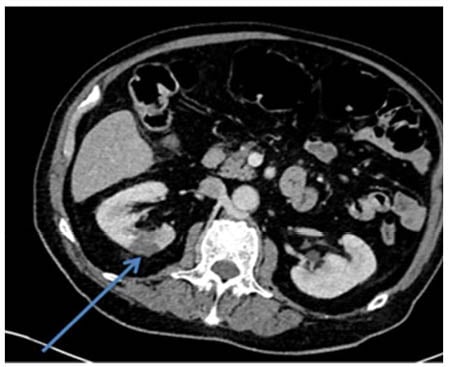
Figure 1: CT-arterial portography.
Day 18 (one slice): wedge infarct within the interpolar segment of the right kidney. This can be seen as a hypodense wedge-shaped region labelled by the arrow.
Unilateral Visual Loss: Right Central Retinal Artery Occlusion
On Day 18, they also reported a 2-day history of right eye visual loss; an ophthalmologist advised likely central retinal artery occlusion but as it was >24 hours since onset of visual loss, no acute intervention was indicated. A CT of the brain demonstrated no acute cortical infarct but some hyperdensity along the interhemispheric falx. With specialist advice from neurology and haematology, their enoxaparin was increased from 40 mg twice daily (as per hospital protocol [enhanced prophylaxis in COVID-19]) to an enhanced treatment dose of 150 mg once daily (normal treatment dose being 1.5 mg/kg; 120 mg in this case) and aspirin 75 mg was commenced. A CT venogram demonstrated a complete filling defect in the right internal carotid artery. Neurosurgeons advised it was likely longstanding and he was not on any medical interventions.
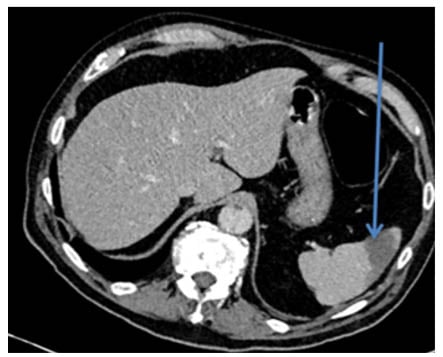
Figure 2: CT-arterial portography.
Day 18 (one slice): one of the splenic infarcts. An arrow labels a hypodense region in the spleen. The hypodense region represents infarcted splenic tissue.
In light of the thromboembolic events, it was pertinent to consider possible sources. Serial blood cultures grew gram negative rods (Bacteroides ovatus) on Day 19 of symptoms. Dental sources were ruled out. As per microbiology, no antibiotics were commenced. A transthoracic echocardiogram was normal, with no valvular abnormalities. A full rheumatological screen was negative.
Bilateral Foot Pain: Bilateral Popliteal Artery Occlusions
On Day 20 of symptoms, the patient developed bilateral cold, painful feet with dusky discoloration of their toes and absent pulses distally to the popliteal arteries. An urgent ultrasound with Doppler flow demonstrated complete occlusion of the left superficial femoral artery and the right popliteal artery (Figure 3). Enhanced treatment dose enoxaparin was switched to a heparin infusion and the patient was transferred to a vascular surgery centre.
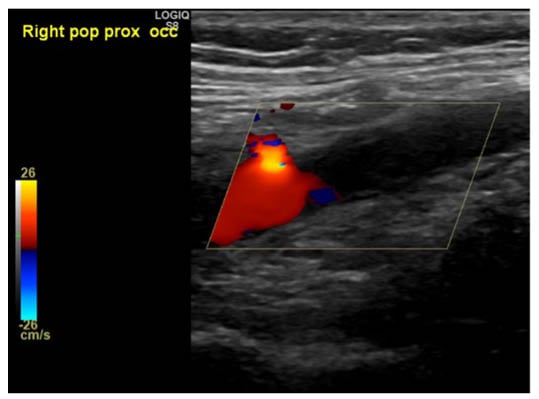
Figure 3: Ultrasound right lower limb with Doppler flow.
Popliteal artery occlusion noted; no flow in the calf arteries demonstrated more distally.
Blood results had also worsened, with a WCC of 38×109 /L, a D-dimer of 3,800 ng/mL, a ferritin of 5,800 μg/L, and a blood film showed neutrophilia with toxic granulations and platelet clumps.
Bleeding per Rectum: Ischaemic Colitis
The patient had two episodes of malaena on arrival at the vascular centre, with new onset fast atrial fibrillation and abdominal tenderness. A triple phase CT with CT angiogram in the emergency department demonstrated free floating thrombus in the proximal left subclavian artery and aortic mural thrombi at multiple levels; embolic thrombi within the distal tributaries of the superior mesenteric artery, likely to account for the ischaemic colitis, was also seen. Also noted was a grossly oedematous caecum with haemorrhagic content inside the bowel wall. There was no active contrast extravasation; however, a contained perforation of the caecum and appendix was likely. Unchanged ischaemic insults to the right kidney and spleen with no pulmonary emboli were also noted; however, persisting ground glass opacification and organised pneumonia was seen.
These features represent multiple ischaemic events secondary to a catastrophic thromboembolic storm (also referred to as red rain).
Surgery: Colectomy and Embolectomy
On Day 21, the patient underwent a right hemicolectomy, which showed extensive ascending colon infarction and caecal perforation. This was followed by bilateral lower limb embolectomies and transverse popliteal arteriotomies. Although significant fresh thrombii alongside inflammatory material were retrieved, by this time, irreversible ischaemia with fixed mottling had developed across his toes.
Outcome
Unfortunately, intra-operative deterioration required both increasing inotropic requirements and ventilator support. The patient was transferred to the intensive care unit but sadly succumbed to the burden of disease on Day 22.
DISCUSSION
Consequences of acute COVID-19 infection are far-reaching and can impact all organ systems. As a relatively new virus, the impact on the respiratory system is well appreciated, has been extensively studied, and reported. However, it has become increasingly apparent that COVID-19 is more than a chest infection, with wide ranging multi-system effects and systemic inflammation reported in several organs, including the kidneys, brain, and gut. The understanding, identification, and treatment of thromboembolic disease, particularly pulmonary embolism, has significantly improved since the first wave of COVID-19 infection, experienced in the UK in March–April 2020. Despite sequential uptitration of anticoagulation, from enhanced thrombo-prophylaxis (enoxaparin 40 mg twice daily), to enhanced treatment dose (enoxaparin 150 mg once daily), to a heparin infusion, the authors patient progressed to thromboembolic storm.
A recent publication discussed the mechanisms behind the increased thrombosis leading to increased mortality and morbidity.1 The mechanisms included immune-mediated thrombotic mechanisms; complement activation; macrophage activation syndrome; antiphospholipid antibody syndrome; hyperferritinaemia; and renin-angiotensin system dysregulation.
In addition, severe damage to endothelial cells in COVID-19 results in coagulation activation.2 The RECOVERY trial by Oxford University, UK, concluded that tocilizumab (often used in rheumatoid arthritis) reduced deaths in patients hospitalised with COVID-19.3 Coagulation activation could be more common in a patient with an underlying inflammatory condition, such as rheumatoid arthritis in this case.
Neutrophil extracellular traps formation is a feature of COVID-19 infection.4 The marked neutrophilia in this patient is very likely to have contributed to the appearance of neutrophil extracellular traps, which are essential for immune-mediated thrombosis.5 Detection of neutrophil extracellular traps markers (such as cell-free DNA and myeloperoxidase) in a patient’s plasma at several time points during a COVID-19 infection may be beneficial for patient outcomes in the future. Unfortunately, the authors do not have access to stored patient plasma, which is a limitation of this manuscript.
The mechanism behind the thromboembolic storm episode requires further research. The thrombotic episodes tend to be proportional to the severity of COVID-19 infection with an incidence of 31% in patients admitted to the intensive care unit.6
Pre-publication, interim results from a multiplatform, international, randomised control trial (ATTACC, ACTIV-4A, and REMAP-CAP),7 suggest that the timing of anticoagulant therapy could be vital in COVID-19. It has concluded that therapeutic dose heparin reduces mortality when initiated in individuals with moderate infection; and when an individual is severely ill, there is a 98.5% probability that initiating treatment dose heparin is harmful when compared to thromboprophylaxis.
Furthermore, the potentials of thrombolytic therapy could be considered in the future.1 Specialist input from haematology with continuously updated guidelines, protocols, and individual case management, is vital in patients with COVID-19. With the increasing prevalence and understanding of thromboembolic disease, international guidance for the prevention and management of thromboembolism is essential for best practice in clinical decision-making processes.
Other case reports detailing thromboembolic storms in COVID-19 exist.8 Culminating information and data across the globe is fundamental to developing the scientific and medical knowledge; knowledge will result in more effective treatment of an acute COVID-19 infection.
TAKE HOME MESSAGES
- To be aware of varied and evolving presentations of moderate-to-severe COVID-19.
- Keep a low threshold of suspicion for thromboemboli in moderate-to-severe infection.
- Ongoing grading of severity of infection and appropriate dosing of anticoagulant therapy is vital.
- Coagulation activation in those with underlying inflammatory conditions could result in increased morbidity and mortality and, therefore, need to be explored as part of an anti-coagulation protocol.
PATIENT’S PERSPECTIVE
‘Deeply shocking’ is the sentiment that the patient’s partner wanted to portray to readers. On admission, their fully fit partner could walk for miles a day. The patient subsequently deteriorated, despite a lack in oxygen requirements. Furthermore, the patient was due to have their vaccine dose on the day that they sadly passed away. With the roll-out of a global vaccination programme, these deaths can be potentially prevented. Finally, the patient’s partner would like to extend their gratitude to the entire healthcare service, unselfishly working throughout the pandemic.








