Abstract
Lemierre’s syndrome (LS) is a rare but potentially life-threatening condition resulting from oropharyngeal infections. It is characterised by septic thrombophlebitis of the internal jugular vein and disseminated metastatic abscesses. However, atypical presentations with unusual sites of thrombosis and spread have been reported. The authors present a case of LS with an atypical presentation in a previously healthy 17-year-old male. This case highlights the need for a high level of suspicion, and comprehensive investigation in cases of unexplained sepsis following oropharyngeal infections, as LS can have atypical presentations and potentially life-threatening complications. The traditional definition of LS may need to be re-evaluated in light of such atypical manifestations.
Key Points
1. This case report highlights Lemierre’s syndrome (LS), a rare but serious condition arising from oropharyngeal infections, highlighting the necessity of increased awareness among clinicians to prevent severe outcomes.2. The article details a case of atypical LS, characterised by unusual thrombosis sites and septic complications, underscoring the importance of comprehensive investigations and an extensive differential in cases of unexplained sepsis.
3. Clinicians should consider LS in differential diagnoses of sepsis following pharyngitis, particularly with atypical presentations. Early recognition and intensive treatment are crucial to improving patient outcomes in such potentially fatal conditions.
CASE HISTORY
A previously healthy 17-year-old male was transferred from a community hospital to the authors’ tertiary paediatric emergency department due to concern for sepsis. On presentation to the community hospital, the patient was febrile, tachycardic, and hypotensive. Before transfer, he was resuscitated with intravenous fluid boluses and was given a dose of ceftriaxone. Further history revealed that in the preceding 5 days, the patient had fevers with chills, sore throat, odynophagia with associated trismus, emesis, and right-sided groin pain. His past medical history was unremarkable, with no recent travel or sick contacts. He disclosed recent marijuana and tobacco use, and ongoing contact with four Rottweilers. His immunisations were complete up until 12 years of age; however, he was unimmunised for COVID-19. Upon admission to the authors’ hospital, the patient remained mildly tachycardic but was haemodynamically stable. General examination revealed a few small and tender lymph nodes in the anterior cervical chain (<1.5 cm), along with an area of tenderness at the left angle of the mandible. Oropharyngeal examination showed Grade 1 tonsils (tonsils just outside of the tonsillar fossa and occupying ≤25% of the oropharyngeal width) bilaterally.1 The patient had a normal cervical range of motion, but limited jaw opening of two to three finger breadths. Abdominal examination revealed mild tenderness in the epigastric and right inguinal region with no associated masses. The neurological examination was unremarkable.
DIFFERENTIAL DIAGNOSIS AND INVESTIGATIONS
Laboratory examinations showed a normal leukocyte count (9.5×109 cells/L), elevated c-reactive protein (270 mg/L), thrombocytopenia (36×109 /L), elevated D-dimer assay (3,226 µg/L), hyponatraemia (126 mEq/L), and high creatinine (147 µmol/L). Broad-spectrum intravenous antibiotics were initiated empirically including ceftriaxone, clindamycin, and vancomycin due to the concern for sepsis and potential toxic shock syndrome. Methicillin-resistant Staphylococcus aureus screen; sexually transmitted infection screen including HIV, syphilis, gonorrhoea, chlamydia, and hepatitis B; and serology assays for COVID-19, hepatitis B virus, hepatitis C, cytomegalovirus, Epstein–Barr virus, toxoplasmosis, Lyme, and parvovirus were all negative. Initial neck ultrasound and chest radiograph on presentation were unremarkable. Blood, throat, and urine cultures (collected after starting antibiotics) were negative.
Considering the patient’s initial presentation with neck stiffness and trismus, there was a strong suspicion of a potential deep neck pathology. On admission, a head and neck CT scan revealed left retromandibular vein thrombosis and infiltrative densities, suggestive of thrombophlebitis. A repeat ultrasound and Doppler study of the neck also revealed similar findings (Figure 1 and Figure 2). Based on these findings, a presumptive diagnosis of Lemierre’s syndrome (LS) was made, and further imaging was initiated. MRI of the head showed punctate diffusion restriction of the right frontal–parietal subcortical white matter, suggestive of cerebral infarction. Persistent right groin pain prompted further radiological investigations. CT abdomen/pelvis showed a moderate amount of non-specific ascites. Subsequent MRI of the pelvis identified a right iliacus abscess measuring 2.7 cm x 3.8 cm, confined to the iliacus musculature without extension to adjacent muscles or bones (Figure 3 and Figure 4). Culture of the fluid drained from the abscess via ultrasound-guided aspiration, while on antibiotics, was sterile. 16S ribosomal RNA (16S rRNA) PCR was also performed and confirmed the presence of Fusobacterium necrophorum. A cardiac bubble study revealed normal cardiac anatomy with no shunting. This constellation of clinical sepsis, a history of pharyngo-tonsillitis, thrombus of left retromandibular vein, acute cerebral infarct, and F. necrophorum pelvic abscess confirmed the authors’ working clinical diagnosis of LS.
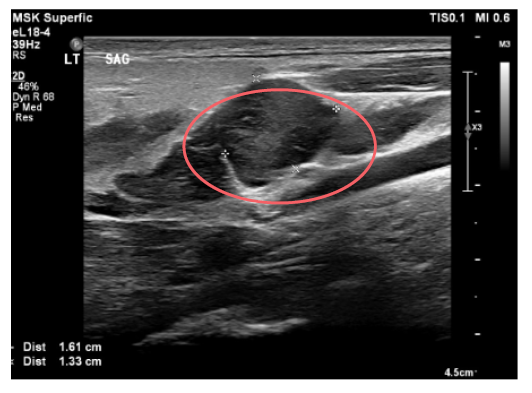
Figure 1: Occlusive retromandibular vein on ultrasonography.
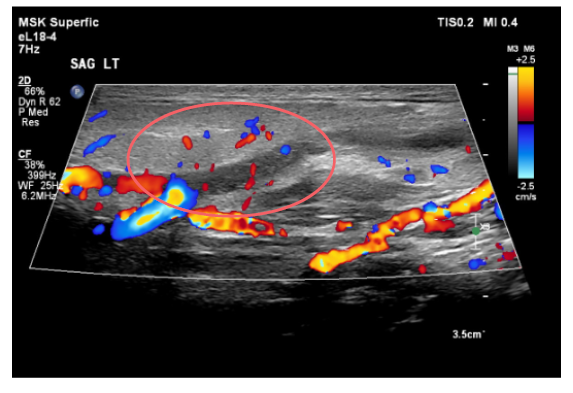
Figure 2: Doppler image shows lack of flow through the retromandibular vein.
TREATMENT, OUTCOME, AND FOLLOW-UP
Following the clinical diagnosis of LS, antibiotic therapy was adjusted to ceftriaxone and metronidazole. Anticoagulation therapy with enoxaparin was initiated and later transitioned to rivaroxaban. The patient experienced a favourable recovery throughout his hospital stay, with significant clinical and biochemical improvement during his admission. Despite the radiologically significant cerebral infarction on MRI, the patient did not present with any neurological deficits clinically. Paediatric neurology assessed the patient during admission, reviewed the MRI findings, and confirmed that imaging findings were clinically non-significant. He was discharged on the 12th day of admission with a prescription for intravenous ceftriaxone, administered via a peripherally inserted central catheter line, to complete a total course of 6 weeks. Additionally, the patient received anticoagulation therapy for a duration of 3 months. At the 2-month follow-up, a neck ultrasound revealed a stable appearance of the thrombus, and the patient exhibited satisfactory clinical progress. Other follow-up laboratory investigations, including complete blood count and c-reactive protein, were normal.
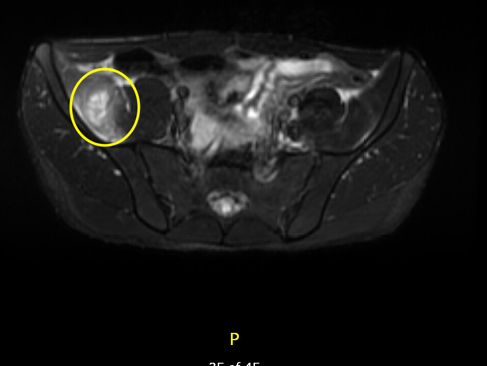
Figure 3: Axial view on MRI pelvis showing right iliacus abscess.
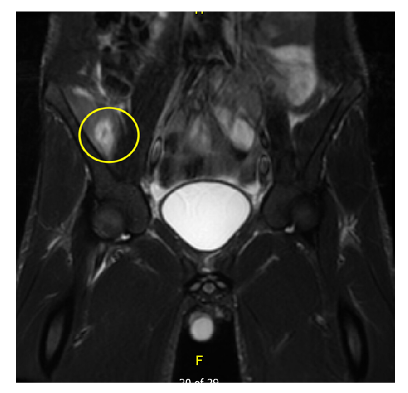
Figure 4: Coronal view on MRI pelvis showing right iliacus abscess.
Three months post-discharge, the patient re-presented to the emergency department with fever, neck pain, sore throat, and emesis. A repeat neck ultrasound revealed a new left internal jugular vein (IJV) thrombus. He underwent a septic workup, including blood cultures and a CT neck, which were negative for an infectious cause of his symptoms. It was determined that he likely had a viral illness in addition to the new left IJV thrombus. Additionally, it was discovered that the patient had not been adherent to his medication prior to this presentation. Two months after the second hospitalisation, and with consistent adherence to his anticoagulation therapy, a repeat ultrasound showed complete resolution of the left IJV thrombus. Consequently, the patient was discharged from the paediatric haematology clinic.
DISCUSSION
LS, often regarded as the ‘forgotten’ disease, is a rare but potentially life-threatening illness that primarily affects healthy young adults and adolescents.2 It typically arises from oropharyngeal infections, leading to thrombophlebitis of the IJV and distant metastatic abscesses. LS impacts the pulmonary system with pulmonary abscesses in 95% of the patients, which is often considered a key diagnostic criterion.3,4 The most common causative organism in LS is F. necrophorum, although other pathogens such as β-haemolytic streptococci have also been rarely reported.2,5 Atypical sites of infection associated with LS have been reported in the abdomen, often in association with urogenital infections,6 or with pelvic infections.7 While rare, CNS complications in LS such as meningitis and cerebral abscess have been reported.8,9 In the authors’ case, the progression of the disease had atypical features. The site of thrombosis was the left retromandibular vein, and the patient developed a right iliacus abscess and cerebral infarction as metastatic complications.
In the case the authors presented, the occurrence of an iliacus abscess away from the primary cervical infection site is hypothesised to result from hematogenous spread rather than local extension. This hypothesis is supported by the absence of pelvic or abdominal infections that have been found to precede such abscesses,10 coupled with the identification of septic emboli in cervical veins, suggesting a possible route for pathogens to reach distant sites such as the iliacus muscle. This scenario is consistent with other rare reports in the literature where distant muscular abscesses developed in the absence of adjacent primary infections, highlighting the potential for hematogenous spread in cases of LS. Such cases highlight the importance of considering atypical infectious pathways in systemic infections, especially when clinical presentations seem to deviate from expected norms.11 Also, in the authors’ patient, the occurrence of cerebral infarction raises the question of its aetiology in the context of LS. While typically associated with septic emboli,9 there was no definitive evidence suggesting bacterial seeding, or abscess formation in the brain. Instead, the authors hypothesise that the hyper-coagulable state induced by systemic inflammation and infection could have contributed to ischaemic stroke.12 This view is supported by the elevated inflammatory markers during the course of the patient’s illness and absence of venous-arterial shunts on imaging.
A search on PubMed for the past 20 years using the following terms: “cerebral infarction+Lemierre’s syndrome” and “iliacus muscle abscess+Lemierre’s syndrome,” yielded only four case reports of cerebral infarction, with only one case reported in the paediatric population. Additionally, only one case report discussed an iliacus muscle abscess in relation to LS. Table 1 reports the findings. The literature review was deliberately focused on using specific search terms to correlate directly with the atypical manifestations observed in the authors’ case. This targeted approach was chosen to ensure a thorough exploration of the most relevant studies, enabling a detailed and appropriate discussion on the rare occurrences of cerebral infarction and iliacus muscle abscess in LS.
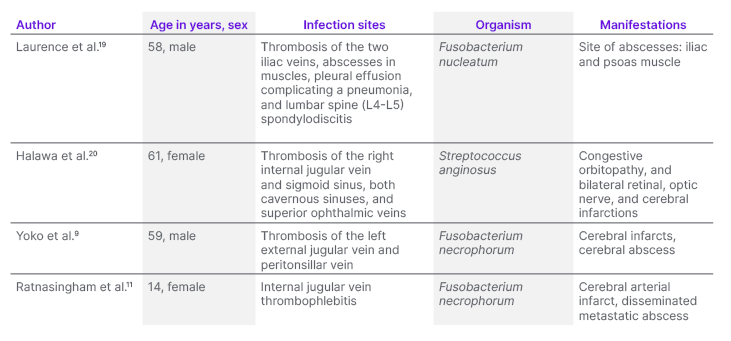
Table 1: Case reports of atypical Lemierre’s syndrome with cerebral infarction and/or iliacus muscle abscess.
Bird et al.13 conducted a systemic review on 78 cases of LS, challenging the traditional understanding of the condition. Their findings revealed that approximately 21% of cases had primary infections in non-oropharyngeal sites, 31% of cases did not exhibit IJV thrombosis, and 30% lacked any pulmonary involvement. Based on these observations, the authors proposed redefining LS as F. necrophorum sepsis, incorporating thrombosis as an additional diagnostic criterion. While septic metastases are commonly associated with LS, and display significant anatomical variation, they are not essential for diagnosing the condition. The mainstay of LS treatment is prolonged broad-spectrum antibiotic therapy, typically administered for around 6 weeks, and guided by clinical response.14 Early diagnosis and antimicrobial therapy have significantly improved the prognosis of LS compared to the pre-antibiotic era, when fatality rates were as high as 5%.15-17 The use of anticoagulant therapy in LS remains controversial.18 Due to the rarity of the condition, and the lack of randomised controlled studies, there are currently no definitive recommendations for the use of anticoagulation in LS. However, in high-risk patients with severe IJV thrombosis or septic spread despite antibiotic therapy, anticoagulation therapy can be considered.18 Given that the patient developed complications that were likely due to septic emboli, the authors opted to initiate anticoagulation alongside antimicrobial therapy.
Diagnosing LS can be challenging as it often occurs when F. necrophorum is incidentally isolated in patients, leading to significant delays in appropriate care.21 As witnessed in the authors’ patient, a high index of clinical suspicion is crucial for the prompt diagnosis and treatment of LS. Early signs of acute pharyngitis may not be sufficient to identify septicaemia and other potentially life-threatening sequelae.2 While documenting a positive culture with a classical pathogen is an important diagnostic feature of LS, F. necrophorum cultures can take 6–8 days to grow.21 Blood cultures in LS have limited sensitivity, particularly when abscesses are present.22 In such cases, molecular diagnostics, such as 16S rRNA testing, can aid in identifying bacterial pathogens. This technique is increasingly used as a promising supplemental diagnostic test.23 This molecular diagnostic tool is not specifically targeted to F. necrophorum, and has broad applications in detecting a variety of bacterial pathogens from different sample types, including serum and directly from abscesses. Results from this method are generally available within a few days.23 Although not routinely used, 16S rRNA might be particularly helpful in identifying the causative organism where cultures remain negative, as was observed in the authors’ patient, confirming the diagnosis and guiding antimicrobial therapy.24
CONCLUSION
The case discussed here emphasises the importance of considering rare complications of common oropharyngeal infections. Unexplained sepsis in a previously healthy young adult, with a recent history of pharyngitis or tonsillitis, should raise suspicion of LS, despite the nonspecific clinical presentation and rarity. Therefore, maintaining a high index of suspicion is crucial. Although uncommon, the identification of septic cervical venous thrombosis in locations other than IJV should prompt a comprehensive investigation for additional metastatic infections. LS carries the potential to cause fatality, and because of this, healthcare providers should be aware of it, and be prompted to recognise it. Furthermore, the presence of atypical metastatic disease, and the frequently reported absence of IJV thrombosis underscores the need for a re-evaluation of the traditional definition of LS, considering these variations in presentation.
KEY CLINICAL MESSAGE
This article aims to educate readers about Lemierre’s Syndrome (LS), emphasising its clinical features, including oropharyngeal infections and thrombophlebitis, as well as challenges in diagnosis. It also highlights uncommon LS presentations like cerebral infarctions and non-typical abscess locations, underlining their significance. Additionally, it discusses the limitations of traditional culture methods, and the potential value of 16S rRNA testing for identifying causative pathogens when cultures yield no results.







