Abstract
Distinguishing neurodegenerative from primary psychiatric conditions is often challenging for clinicians, particularly when assessing older people presenting with neuropsychiatric symptoms. Measurement of fluid biomarkers of neurodegeneration is an emerging approach offering improved diagnostic accuracy. This report explores the use of emerging fluid biomarkers to address diagnostic challenges, framed around a case where the diagnosis of delirium with dementia was revised based on biomarker analysis, enabling treatment of a primary mood disorder with disabling psychiatric symptoms.
Key points
1. Overlap in the clinical presentations for psychiatric conditions, delirium related to underlying dementia, and early behavioural variant frontotemporal dementia create diagnostic challenges, particularly for older adults. Misdiagnosis may lead to inappropriate treatment and related complications.
2. This case report demonstrates how the measurement of neurodegeneration-associated fluid biomarkers can markedly improve the accuracy and timeliness of diagnosis for patients presenting with neuropsychiatric symptoms and diagnostic uncertainty.
3. Clinical studies measuring cerebrospinal fluid and blood neurofilament light levels demonstrate its power to distinguish between primary psychiatric and neurodegenerative conditions; therefore, neurofilament light is a promising candidate for translation to clinicians’ diagnostic ‘toolkits’ in the near future.
INTRODUCTION
Measurement of fluid biomarkers of neurodegeneration is an emerging approach offering improved diagnostic accuracy of neuropsychiatric conditions, particularly for atypical presentations. This article reports a case where the diagnosis of delirium with dementia was revised based on biomarker analysis, enabling treatment of a primary mood disorder with resolution of recurrent disabling psychiatric symptoms.
CASE DESCRIPTION
A 75-year-old male was admitted to a tertiary Australian hospital’s acute medical unit with 3 weeks of progressive agitation and paranoia. This was on a background of multiple similar admissions for hyperactive delirium and behavioural variant frontotemporal dementia (bvFTD) clinically diagnosed 2 years prior. Other medical history included ischaemic heart disease, atrial fibrillation, hypertension, and dyslipidaemia. They had no psychiatric history.
Extensive delirium work-up, including blood and urine analysis, neuroimaging (MRI), electroencephalography, and cerebrospinal fluid (CSF) analysis for infective or autoimmune conditions, failed to reveal a precipitant. Despite care in a specialised delirium management ward for over a week, and treatment with intramuscular haloperidol, they remained agitated, disinhibited, and paranoid. Psychiatric evaluation deemed a primary psychiatric disorder to be unlikely, given the consistency of this presentation with previous delirium episodes.
However, careful review of previous admissions, and assessments from interceding outpatient Memory Clinic appointments, revealed three similar episodes lasting 3–6 weeks over the preceding 2 years, with complete symptom resolution between episodes. Objective cognitive assessments, repeated between episodes and during the current admission, were stable (Montreal Cognitive Assessment [MoCA] score: 25/30). Outpatient neuropsychological evaluation also demonstrated stable cognitive performance within the normal range, inconsistent with dementia.
Ongoing diagnostic uncertainty prompted further CSF analysis. Biomarker assay results for Alzheimer’s disease1 (amyloid β1–42 and phosphorylated tau) and general neurodegeneration (neurofilament light [NfL] and total tau) are given in Table 1. While amyloid β1-42 levels were decreased, total tau and phosphorylated tau levels were normal. Absence of marked NfL elevation, despite 2 years of severe neuropsychiatric symptoms, argued strongly against underlying neurodegenerative pathology such as bvFTD. In light of this, reassessment of the patient’s mental state and review of their clinical trajectory by the acute hospital Old Age Psychiatry consultation service resulted in a revised diagnosis of bipolar affective disorder. This facilitated referral to an inpatient psychiatric hospital, where treatment with lithium and quetiapine achieved long-term remission, sustained for 3 years.
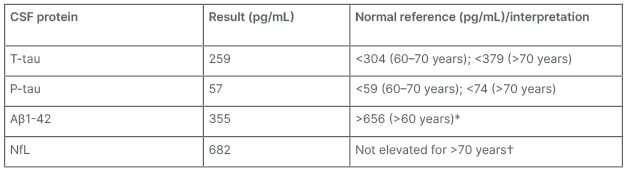
Table 1: Biomarker assay results for Alzheimer’s disease.
CSF biomarker results profile was not consistent with neurodegeneration or Alzheimer’s disease-
related pathology.
*An isolated reduced Aβ1–42 can be a non-specific finding in in many neurodegenerative and non-
neurodegenerative conditions and may normalise after acute episodes. Therefore, in the absence of
abnormalities in t-tau and p-tau, low Aβ1–42 was not considered to be suggestive of an Alzheimer’s disease-related pathology.
†Age-normative ranges for NfL remain to be validated for routine clinical use. This result was compared against a contemporaneous research population involving patients with psychiatric and neurodegenerative disorders, for which the same analytical technique was employed.2 The optimal research cut-off for distinguishing neurodegenerative from primary psychiatric and non-neurodegenerative disorders in people aged >70 years was 970 pg/mL (from this research study cohort).
Note: NfL concentrations were measured in duplicate using a commercial ELISA (NF-Light™ [UmanDiagnostics, Umeå, Sweden]), according to the manufacturer’s protocol.
Aβ1–42: amyloid β1–42; CSF: cerebrospinal fluid; NfL: neurofilament light; p-tau: phosphorylated tau; t-tau: total tau.
DISCUSSION
For patients presenting with neuropsychiatric symptoms, particularly in cases of diagnostic uncertainty as described here, addition of fluid biomarker measurement to the diagnostic toolkit can markedly improve the accuracy and timeliness of diagnosis.
Distinguishing early bvFTD, delirium related to underlying dementia, and psychiatric conditions from one another can be especially challenging due to symptom overlap.3 Misdiagnosis may lead to inappropriate treatment and significant complications, including repeated hospitalisation and antipsychotic administration, as demonstrated by this case. Particularly in older adults, treatment of presumed hyperactive delirium in the context of dementia with antipsychotics carries a risk of significant mortality and morbidity; therefore, rigorous interrogation of atypical presentations is imperative.4,5 Objective disease markers are needed to improve diagnostic accuracy in clinical scenarios such as these.
NfL appears particularly promising as a candidate for clinical application in the near future. Clinical studies using CSF NfL levels demonstrate its power to distinguish between primary psychiatric and neurodegenerative conditions, with a recent study showing high discriminatory accuracy (area under the curve: 0.94; sensitivity: 92%; specificity: 87%).2,6 Given the logistical challenges inherent in CSF sampling, advances allowing reliable measurement of NfL in blood significantly improve the feasibility of rapid translation to the clinic.7 Concordance between CSF and blood NfL levels has been established across a range of neurodegenerative conditions, with subsequent blood-only studies supporting NfL as a useful tool for the discrimination of neurodegenerative and psychiatric diagnoses.8,9
CONCLUSION
Clinicians should be optimistic about the much-needed diagnostic clarity that NfL measurement, in CSF or blood, may offer their patients suffering from neuropsychiatric symptoms.








