Abstract
An estimated 65 million people worldwide have moderate or severe chronic obstructive pulmonary disease (COPD), an umbrella term used to describe a group of progressive lung diseases that obstruct airflow such as emphysema and chronic bronchitis. Smoking contributes to an estimated 90% of COPD cases, as the harmful chemicals produced during tobacco combustion damage the lungs and airways. Although smoking cessation is the only intervention shown to improve COPD prognosis in smokers, many patients who try to quit continue to smoke. The continued use of conventional cigarettes exacerbates COPD symptoms, and globally more than 3 million people die from the disease every year. The last two decades have seen the introduction of combustion-free nicotine delivery alternatives that produce significantly lower levels of the harmful components in cigarette smoke, and researchers have begun to assess the impact of switching from cigarettes to these products. Several studies have examined how patients with COPD use e-cigarettes as assistance for quitting, but few have examined how heated tobacco products (HTP) may reduce risk. This narrative review summarises results from pre-clinical, clinical, and real-world evidence studies showing possible harm reduction benefits for patients with COPD who switch to HTPs rather than continuing to smoke cigarettes. Epidemiological studies, real-world data analyses, and randomised clinical trials must be conducted to determine whether switching from cigarettes to HTPs can improve health outcomes in patients with COPD who would otherwise continue to smoke combustible cigarettes.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a preventable lung condition that represents a major and growing cause of global morbidity and mortality, and is primarily due to cigarette smoking.1,2 The World Health Organization (WHO) cites it as the third leading cause of death after ischaemic heart disease and stroke.3 The mortality risk from COPD is 12–13 times greater in smokers of combustible cigarettes,4 and an estimated 80–90% of patients with COPD smoke(d).5 Beyond the 3 million deaths attributed to COPD annually, the condition also has a major impact on healthcare systems: all smoking-related diseases, including COPD, are responsible for 1.5–6.8% of national health system expenditures worldwide.6
COPD is not a singular respiratory disease; it encompasses a range of treatable (but not curable) chronic lung conditions (including chronic bronchitis and emphysema) characterised by respiratory symptoms, progressively worsening airflow limitation, and resultant airway inflammation. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,7 lung function evaluation via spirometry testing (e.g., forced expiratory volume in one second [FEV1] and forced vital capacity) is the gold standard for diagnosing COPD and tracking disease progression. However, COPD is widely thought to be underdiagnosed, and many current smokers who do not meet the diagnostic standards do have respiratory symptoms without airflow obstruction.7
The symptoms of COPD are induced and exacerbated by exposure to cigarette smoke,8 which causes inflammation and oxidative stress.9 There is no question that smoking cessation is by far the best approach to reduce disease progression in patients of any age. Most subjects with COPD are or were smokers and therefore have first-hand experience with the detrimental effects of cigarettes, but multiple studies reported that roughly half of smokers with COPD attempted to quit smoking in the past year.10-12 Unfortunately, only approximately 20% of patients with COPD are successful for 1 year,13 and many eventually relapse. Considering this small percentage, complementary tobacco harm reduction approaches should also be considered for this specific population of adult smokers.
Chronic Obstructive Pulmonary Disease and Cigarette Smoke
The primary cause of smoking-related disease and mortality is the harmful and potentially harmful constituents (HPHC) formed during tobacco combustion. A burning cigarette releases a complex mixture of ultrafine solid and gaseous particles, and more than 6,000 chemical constituents, approximately 100 of which have been identified by public health authorities as HPHCs linked to smoking-related diseases. These include particulates, oxidising chemicals, polycyclic aromatic hydrocarbons, acrolein, butadiene, metals (e.g., cadmium), and carbon monoxide (CO). These HPHCs are deposited throughout the respiratory tract, leading to cilia toxicity, oxidative stress, inflammation, impairment of lung defences, and irritation.9 Their continued presence causes changes in airway and alveolar cells, and affects tissue functions. This adversely impacts overall pulmonary function, eventually leading to the development of COPD. The most common symptoms are dyspnoea, increased sputum or mucus production, and chronic cough.
Smoke-Free Products
Although the best way to reduce the risks of developing COPD and other smoking-related diseases is to quit tobacco altogether, many people still want to continue using products with similar taste, ritual, and nicotine uptake.14 Recent scientific and technological advances have facilitated the development of innovative smoke-free products (SFP) that have the potential to be less harmful than continued smoking and are acceptable to and may be used by current adult smokers. The most widely used SFPs are e-cigarettes and HTPs. The former vaporises an e-liquid solution when it is puffed, while the latter heats real tobacco. E-cigarettes and HTPs operate differently, but they share the absence of combustion while still providing a satisfactory sensory experience for adult smokers.15-17
HTPs are designed to generate a nicotine-containing aerosol by heating tobacco to temperatures sufficient to release nicotine and flavours from the tobacco (<350 °C), but low enough to prevent the tobacco from burning (400–800 °C in conventional cigarettes).18 As a result, HTP aerosol contains fewer and lower levels of HPHCs than cigarette smoke (Figure 1).19
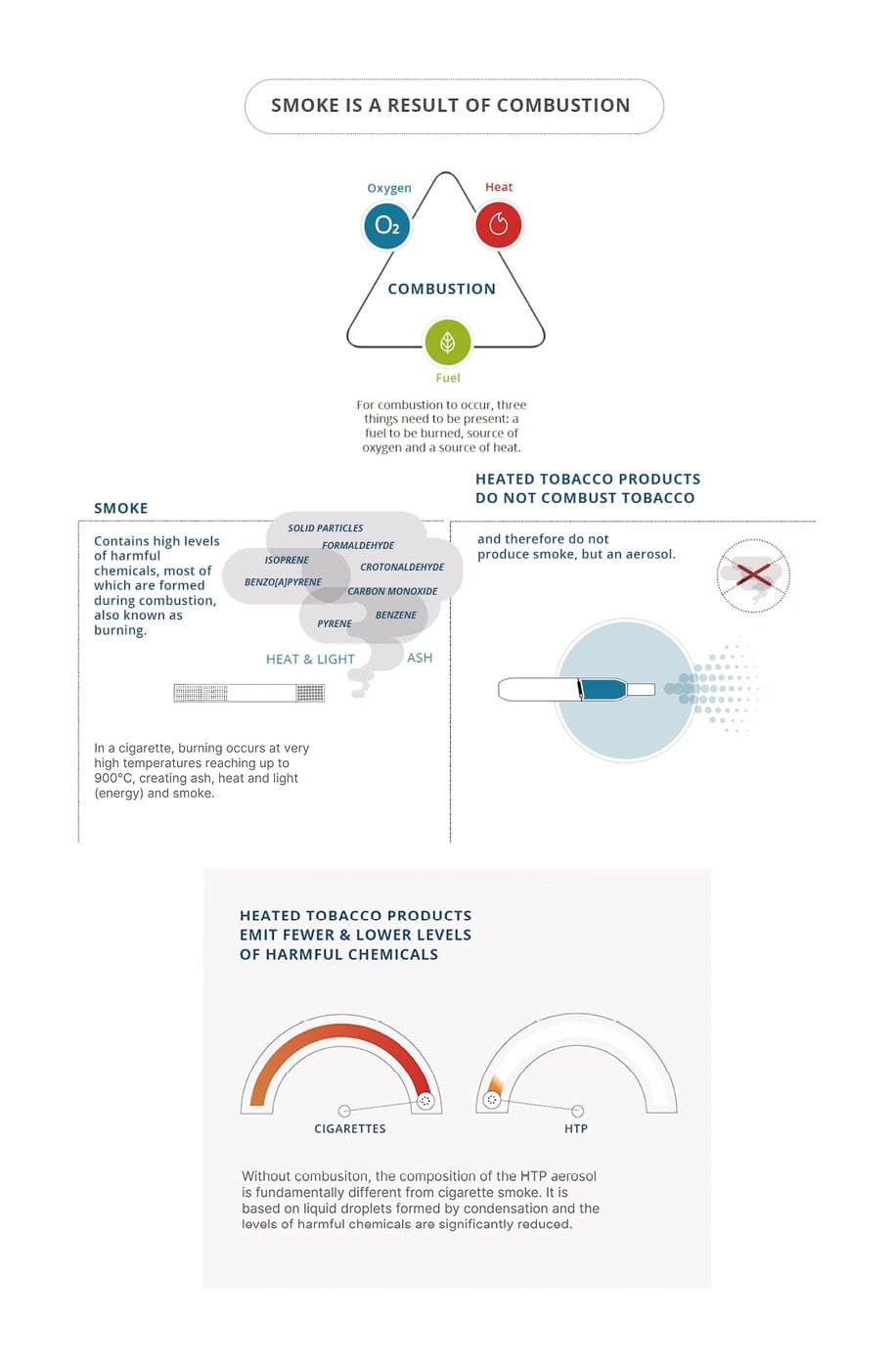
Figure 1: Differences between cigarette smoke and heated tobacco product aerosol.
HTP: heated tobacco product.
Adapted from PMIscience.19
Philip Morris International (PMI, New York, USA) launched the first iteration of the current generation of HTPs in 2014. It was developed as Tobacco Heating System (THS, version number 2.2), commercialised under the IQOS (PMI) brand name, and explicitly marketed to adult smokers unable to quit.20 As of September 2022, it was available in 68 countries. British American Tobacco (BAT, London, UK) launched glo in 2016 and Japan Tobacco (JTI, Tokyo, Japan) launched the HTP Ploom in the same year. Korean Tobacco and Ginseng (KT&G, Daejeon, South Korea) entered the HTP market with the launch of lil in 2017.
APPROACH AND PURPOSE OF THIS NARRATIVE REVIEW
There are a myriad of systematic literature reviews and meta-analyses on the topics of smoking and COPD, with extensive high-level evidence supporting the causal relationship between the habit and disease.13,21-23 Although SFPs are increasingly used by adult smokers, there are fewer studies on the effects of HTPs compared to e-cigarettes. A simple query on PubMed for “e-cigarette” returns 5,495 results, versus 180 for “heated tobacco product.” Some groups have investigated health effects in smokers who switch to e-cigarettes.24,25 Fewer publications are related to HTPs. There is one systematic literature review on heat-not-burn tobacco products,26 and a Cochrane meta-analysis examined the use of HTPs for smoking cessation and reducing smoking prevalence.27 Almost no studies have assessed the individual and population health benefits from tobacco harm reduction for people with COPD and other chronic conditions who switch to SFPs instead of continuing to smoke. As more data are gathered, it is important to understand how alternatives like HTPs may reduce risk in patient populations.
This literature summary contains three sections. The first two describe the studies that support HTP harm reduction based on HPHC levels and a brief overview of pre-clinical findings. These publications are complemented by references to statements from regulatory bodies. The third section describes findings from the above-described literature search that identified published clinical data of HTP effects on HPHC exposure and smoking-related respiratory diseases. The narrative review concludes with a discussion of the cited studies, limitations of the data, and gaps in the literature to be addressed.
The goals of this narrative review were to provide a high-level summary of the studies performed to demonstrate that HPHC levels are lower in HTPs compared to cigarettes; and to identify studies on health outcomes in patients with COPD who switch from combustible cigarettes to HTPs. The publications were identified in the PubMed, Scopus, Embase, Google Scholar, and SciFinder databases using the following search string: (((chronic obstructive pulmonary syndrome[Title/Abstract]) OR (COPD[Title/Abstract])) AND (heated tobacco product[Title/Abstract])) OR (tobacco heating system[Title/Abstract]). The authors also searched the reference lists of relevant reviews and meta-analyses.
LITERATURE SUMMARY
Harmful and Potentially Harmful Constituents Levels
The absence of combustion during THS use and the fact that THS aerosol is not smoke have been validated by leading scientific experts in the fields of combustion, fire safety, and thermochemistry from numerous countries, including Italy, the UK, Japan, Poland, the USA, Australia, Germany, and Switzerland, as well as by an independent research organisation in New Zealand.28
By avoiding combustion, THS generates an aerosol that contains significantly fewer harmful chemicals. Relative to smoke from a reference cigarette, the levels of International Agency Research on Cancer (IARC) Group 1 carcinogens in THS aerosol are reduced on average by >95%.29,30 Compared to cigarettes, THS also emits >94% lower levels of free radicals31 and 85% lower levels of reactive O2 species.32 A 2022 review concluded that SFPs, including e-cigarettes and HTPs, had a reduced effect on oxidative stress compared to cigarette smoking, with the caveat that more investigation is needed to clarify the long-term toxicological impact.33
The significant HPHC reduction in THS aerosol was confirmed by various public health authorities and independent laboratories around the world. In April 2019, the U.S. Food and Drug Administration (FDA) Center for Tobacco Products (CTP) issued a marketing order34 for PMI’s IQOS Tobacco Heating System to allow its introduction in the USA market. In its scientific review of the regulatory application,35 the FDA “found that the aerosol produced by the product under consideration ‘contains fewer toxic chemicals than cigarette smoke’, and many of the toxins identified are present at lower levels than in cigarette smoke. For example, the CO exposure [resulting from this product] is comparable to environmental exposure, and levels of acrolein and formaldehyde are 89–95% and 66–91% lower than from combustible cigarettes, respectively.” In July 2020, the FDA authorised the marketing of IQOS as a modified risk tobacco product with the following information: the IQOS system heats tobacco but does not burn it; this significantly reduces HPHC production; and scientific studies have shown that switching completely from conventional cigarettes to the IQOS system reduces user exposure to HPHCs.36
The German Federal Institute for Risk Assessment (BfR) also conducted an independent analysis of THS and found that the “levels of major carcinogens are markedly reduced in the emissions of the analysed [heat not burn] product in relation to the conventional tobacco cigarettes, and that monitoring these emissions using standardised machine smoking procedures generates reliable and reproducible data, which provide a useful basis to assess exposure and human health risks.”37
Published research on existing HTPs available in various markets confirms the reduction in the number and levels of HPHCs.38-42 This is in line with the statement from Public Health England (PHE, now under the UKHSA) that, “compared with cigarette smoke, heated tobacco products are likely to expose users and bystanders to lower levels of particulate matter, and harmful and potentially harmful compounds. The extent of the reduction found varies between studies.” In addition, “[t]he available evidence suggests that heated tobacco products may be considerably less harmful than tobacco cigarettes, and more harmful than e-cigarettes.”43 However, it is important to note that this potential HPHC reduction should be assessed on a product-by-product basis.
Pre-Clinical Findings
COPD is characterised by airway inflammation, damage, and remodelling.1,4 High levels of HPHCs in cigarette smoke irritate the lungs, introduce free radicals, and stimulate the production of proinflammatory chemokines and cytokines.10 Several in vivo studies have shown that the reduced exposure to HPHCs following THS use translates into lower levels of biomarkers of exposure and significantly reduced biological impacts on the rodent respiratory tract.44-47 These effects of lower exposure manifest as reduced disturbances in biological processes associated with COPD (including oxidative stress, inflammation, and apoptosis), less severe adaptive changes in respiratory tissues, absence of alveolar destruction (emphysema), and reduced pulmonary dysfunction.44,47 Emma et al.33 recently published a detailed review on how HTPs may also impact oxidative stress. They concluded that HTPs appear to have a better safety profile than cigarettes but suggested further long-term studies.33
While aerosols from HTPs appear to be less harmful than cigarette smoke, they may still induce changes. One group reported that HTP exposure induced apoptosis-mediated pulmonary emphysema in mouse lungs.48 Another recent study concluded that HTP aerosol altered rat ultrastructural lung airways and DNA.49 Gu et al.50 found that mice exposed to HTP aerosol exposure for 24 weeks had increased proinflammatory cytokine levels in the lung, impaired pulmonary function, and lung tissue damage. However, many of these changes were not as significant as those induced by cigarette smoke.48-50
Clinical Findings
Human clinical trials with THS indicate that reduced HPHC exposure may have a lower impact on smokers’ health compared to continued combustible cigarette use. However, most studies focused on measuring biomarkers of exposure to toxicants or potential harm as proxies to assess cardiovascular and respiratory disease, and cancer.51 Only a handful of publications have actually examined lung function in smokers. In a clinical trial in Japan, healthy smokers who were not willing to quit switched from menthol cigarettes to menthol THS for 5 days in confinement and 85 days in ambulatory settings, and showed improved lung function (i.e., higher FEV1), similar to that measured in subjects who stopped smoking.52 The authors proposed that this was due to decreased lung inflammation after switching from cigarettes. In a 6-month clinical study in the USA, smokers who switched from smoking cigarettes to predominant THS use showed significant improvements in clinical risk endpoints associated with O2 delivery (decreased carboxyhaemoglobin levels), inflammation (reduced white blood cell count), and COPD (increased FEV1), although their plasma nicotine levels remained similar to that in smokers.53
A longitudinal epidemiological study54 of a Kazakhstani population showed that adult smokers who switched to HTPs showed less decline in health-related parameters than subjects who continued to smoke combustible cigarettes after 1, 2, and 4 years of follow-up. Although the study did not specifically assess subjects with COPD, the cohort included individuals aged 40–59 years with a minimum 10 pack-year smoking history. Smokers who switched to HTPs had fewer respiratory symptoms, better lung function (forced vital capacity and FEV1) and physical exercise capacity (assessed with a 6 Minute Walk Test [6MWT]), and healthier metabolic syndrome parameters (waist circumference, high-density lipoprotein cholesterol levels, and systolic blood pressure) than cigarette smokers.55-57
One group specifically analysed data from a COPD cohort for 3 years after smokers with COPD switched to HTPs. Despite the small number of patients in their study (n=38), Polosa et al.58 found that patients with COPD using HTPs experienced fewer COPD exacerbations and significant improvements in symptoms, exercise capacity, and overall health-related quality of life than patients who continued to smoke cigarettes.58 Importantly, the authors performed a subgroup analysis of dual users who continued to smoke combustible cigarettes in addition to HTPs (n=8) and found a marked reduction in the number of cigarettes smoked per day (confirmed by exhaled CO measurement).58,59 A cross-sectional study from the same research group60 analysed mucociliary clearance, a known biomarker of early respiratory health changes, by measuring the saccharin transit time after SFP use. The median saccharin transit time was almost twice as long in cigarette smokers (13.15 min) compared to never (7.24 min) and former smokers who had abstained for at least 3–6 months (7.26 min). Subjects in the HTP study group had a saccharin transit time (8.00 min), similar to former and never smokers. Based on these findings, the authors concluded that switching from cigarettes to HTPs has a beneficial impact on mucociliary clearance, which could be considered an early respiratory health change.
After their introduction to Japan in 2014, the prevalence of HTP use increased 50-fold from 2015–2019, from 0.2% to 11.3%.61 This coincided with a rapid decrease in cigarette sales in the country, from 186.2 billion sticks in 2014 to 120.9 billion in 2019.62 A recent real-world evidence study analysed hospitalisation numbers associated with COPD using data from the Japanese Medical Data Center (JMDC) over this same time period.63 Careful evaluation of all available data from the JMDC database revealed a significant reduction in the number of hospitalisations for COPD and a non-significant reduction in hospitalisations for COPD plus lower respiratory tract infections after the 2014 THS introduction (Figure 2).
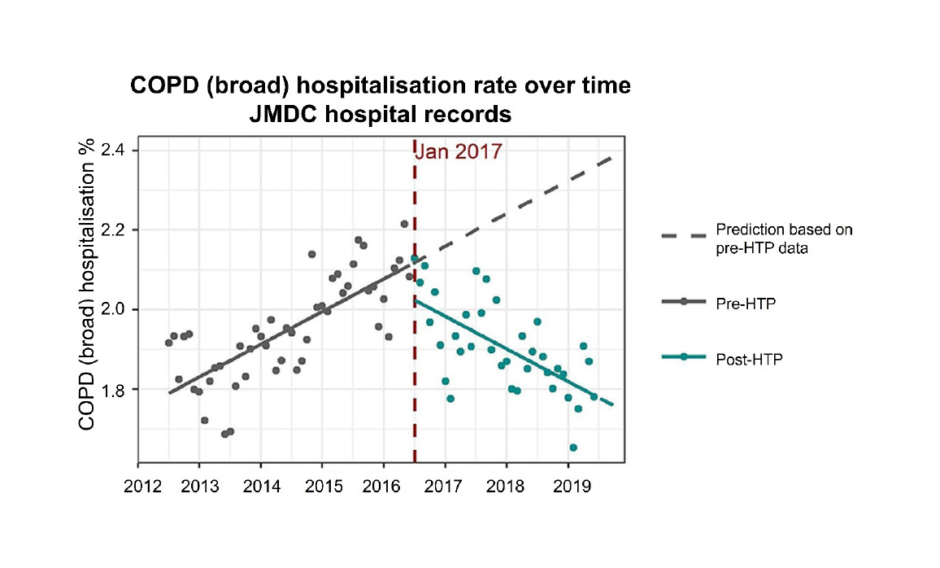
Figure 2: Chronic obstructive pulmonary disease hospitalisation rate over time in Japan (data acquired from the Japanese Medical Data Center [JMDC] database).
The increasing trend of COPD hospitalisations turned downward in 2017, shortly after the introduction of HTPs to Japan.
COPD: chronic obstructive pulmonary disease; HTP: heated tobacco product; JMDC: Japanese Medical Data Center.
Adapted from van der Plas et al.63
DISCUSSION
Although HTPs have only been marketed for a decade, there is a considerable body of evidence from industry and independent studies that HTPs generate lower levels of HPHCs. A Cochrane meta-analysis on 13 clinical studies involving HTPs concluded that there was moderate-certainty evidence that HTP users are exposed to lower levels of HPHCs than smokers.27 There are also numerous pre-clinical studies supporting their relative safety compared to cigarettes.44-50 However, the clinical picture is less clear. A systematic review on HTP exposure and adverse health effects found just 17 studies investigating the effects of HTPs on human health, most of which were short term.64 The authors’ review of the literature identified a small number of studies55-58 that longitudinally assessed lung function in middle-aged subjects who continued to smoke or switched to HTPs, but they were not specifically recruited from a patient population with COPD. In patient-focused studies, the actual numbers of subjects with lung disease or COPD were very low: n=51 (continued smoking) and n=25 (switched to HTPs) in Sharman et al.57 and n=19 in both arms of Polosa et al.58 Real-world evidence studies such as the publication by van der Plas et al.62 can provide some insight into how these products affect population health, but there are many limitations to this type of research. While the results highlight associations, they cannot be used to infer a causal relationship. Longer studies with different designs will ultimately determine whether these findings reflect a reduction in COPD morbidity.
This narrative review also highlights a significant literature gap regarding HTP use by patients with chronic conditions, including COPD. A recent survey on HTP use by subjects with chronic conditions only included 42 patients with COPD out of 9,008 respondents,64 and 33.3% were using HTPs in conjunction with conventional cigarettes. Clearly, more information is needed to determine if completely switching to HTPs improves the quality of life and health outcomes of patients with COPD who would otherwise continue to smoke. This will require multiple lines of evidence from surveys of patients with chronic conditions, continued surveillance of real-world evidence, and the completion of Phase IV trials that will take several years. As noted in a recent meta-analysis,26 only 11 randomised controlled trials have assessed HTP safety and the median follow-up was just 13 weeks. A clinical trial sponsored by PMI is currently recruiting subjects for a 3-year study to assess the effect of switching from cigarette smoking to THS on disease progression in subjects with mild to moderate COPD with chronic bronchitis;65 the estimated study completion date is June 2027.
It is worth noting that HTPs were only recently authorised for sale in the USA (in 2019) and they are currently only available in a few cities. Presumably, more researchers will study their effects as HTP uptake increases in the USA and around the globe.
CONCLUSIONS
The congruence of recent scientific findings indicates that adult smokers who completely switch to HTPs could have a lower risk of COPD development and progression than those who continue smoking. However, the paucity of studies on this specific topic limit the strength of this conclusion. Further epidemiological studies, additional follow-up of real-world data, and prospective clinical trials are needed to understand the impact of switching to HTPs on health outcomes in COPD.
In closing, it is important to state that HTPs are not risk-free and contain nicotine. Although nicotine is addictive, it is not the primary cause of smoking-related diseases.14 The best choice any smoker can make continues to be quitting cigarettes and nicotine altogether. If they choose to switch to HTPs, they should be informed that dual use with conventional cigarettes is not advised.








