Abstract
Background: Liver biopsy, the gold standard for monitoring of methotrexate-induced liver injury, is associated with significant morbidity and mortality. Transient elastography (TE) has been used as a non-invasive alternative to detect liver stiffness.
Aim: To assess the utility of TE in detecting liver fibrosis in patients with methotrexate use.
Methods: A retrospective chart review was performed for 35 patients referred to the liver clinic for evaluation of suspected methotrexate-induced liver injury. Demographic, clinical, histopathological, and elastographic data were collected and interpreted. Liver stiffness measurement (LSM) and controlled attenuation parameter were recorded from TE results.
Results: Thirty-five patients with a mean age of 58 years, including 23 females (66%), were included. The median LSM by TE was 10.8 kPa and the median controlled attenuation parameter was 303 dB/m. A total of 12 out of 35 patients (34%) had evidence of clinical and pathological advanced fibrosis. Using a cut-off elastography value of 10 kPa, the TE yielded 92% sensitivity and 93% negative predictive value for ruling out methotrexate-induced advanced liver fibrosis. Using a higher LSM cut-off point of kPa ≥15.0, specificity was calculated at 87% and positive predictive value at 80%. Area under the receiver operating characteristic curve was 0.80 (95% confidence interval).
Conclusion: FibroScan® (Echosens, Paris, France) has a high sensitivity and specificity for kPa 10 and 15, respectively, for detecting advanced liver fibrosis in patients on methotrexate.
Key Points
1. Despite its efficacy, long-term methotrexate therapy can lead to liver injury characterised by elevation in hepatic aminotransferases, steatosis, fibrosis, and rarely, cirrhosis.
2. FibroScan, a non-invasive test using transient elastography, estimates liver fibrosis and steatosis by measuring liver stiffness and controlled attenuation parameter.
3. The study underscores the importance of larger, prospective studies to validate the utility of FibroScan in managing methotrexate-induced liver injury, and potentially reducing the need for invasive liver biopsies.
INTRODUCTION
Methotrexate (MTX), an antimetabolite, has a multifarious mechanism of action that most notably includes folate antagonism, effects on adenosine signalling, and modulation of many other cellular proteins and pathways, that overall results in an anti-inflammatory effect.1 MTX is used in rheumatologic disorders (e.g., rheumatoid arthritis), dermatologic conditions (e.g., psoriasis), inflammatory bowel disease, and a multitude of other diseases.
Elevation in hepatic aminotransferases is a well-illustrated side effect of MTX therapy. In patients on low-to-moderate dose long-term MTX, aminotransferase elevation occurs in 15–50% of patients, but is typically mild and self-limiting.2 Long-term therapy can also result in steatosis, fibrosis, and rarely, cirrhosis.2 The mechanism of liver injury is believed to be due to inhibition of DNA and RNA synthesis, causing cellular arrest.2 The mechanism of MTX-induced liver fibrosis is postulated to be due to hepatic accumulation of MTX metabolites, leading to excessive homocysteine generation, oxidative stress, inflammation, and ultimately the activation of hepatic stellate cells forming myofibroblasts and resultant fibrosis.2,3 Structural damage in rat livers exposed to MTX include sinusoidal dilatation, inflammatory cell infiltration, congestion, and hydropic degeneration.4
FibroScan® (Echosens, Paris, France), or transient elastography (TE), is a test used to non-invasively estimate the degree of liver fibrosis and steatosis.5 The test consists of an ultrasound transducer mounted on a vibrator. Vibrations are transmitted to hepatic tissue with propagation of an elastic shear wave.6 The velocity of the shear wave is subsequently measured and expressed in kilopascals (kPa). The kPa corresponds to liver stiffness as sound waves travel faster in media with greater elasticity.7-9 Thus, kPa represents the liver stiffness measurement (LSM). Hepatic steatosis is represented by the controlled attenuation parameter (CAP). The CAP is measured by energy loss as the sound wave passes through the medium, and is expressed in dB/m.10,11
A cross-sectional study by Lertnawapan et al.12 in 2018 found an association between cumulative MTX intake in patients with rheumatoid arthritis and hepatic fibrosis measurement by FibroScan.12 It also showed a statistically significant association with BMI, fatty liver, and alanine transaminase levels. Large prospective studies have previously been conducted in patients with non-alcoholic fatty liver disease to establish cut-offs for the LSM and CAP, corresponding to fibrosis stage and steatosis grade, respectively.13-15 These studies compare FibroScan findings with biopsy proven fibrosis stage and/or steatosis grade. Optimal cut-off points are devised typically through the use of receiver operating characteristics (ROC) curves.
However, the accuracy of FibroScan in measuring MTX-induced fibrosis has not been completely established. The aim of this study is to evaluate the utility of FibroScan in predicting advanced liver fibrosis in patients with a history of MTX use.
METHODS
The authors performed a retrospective study of patients referred to the hepatology clinic at Saint Louis University Hospital, Missouri, USA, between 2016–2021, for estimation of liver disease related to MTX use. The hepatology service utilised FibroScan as an initial non-invasive test to screen for presence of advanced liver disease in the setting of chronic MTX use. Patients with a known history of non-alcoholic fatty liver disease, chronic hepatitis B or hepatitis C, or current alcohol use were excluded. This study (IRB #31760) was approved by the institutional review board of Saint Louis University Hospital, Missouri, USA under Exempt Category 4 and was conducted under the Declaration of Helsinki.
Chart review was performed and data regarding sex; ethnicity; BMI; FibroScan results, including LSM (kPa), and CAP (dB/m); liver biopsy results; and signs of clinical cirrhosis were recorded. The authors also collected data regarding outcomes, including follow-up FibroScans, need for transplant, development of hepatocellular carcinoma, and mortality.
LSM cut-off values of more than 15.0 on TE were used to diagnose advanced fibrosis, whereas a cut-off of <10.0 was used to indicate low probability of advanced fibrosis.12,14 Liver fibrosis staging was performed according to Metavir classification.16 Patients with F3 or F4 were considered to have ‘advanced fibrosis’. Patients without a liver biopsy who had a clinical diagnosis of cirrhosis were also considered to have ‘advanced fibrosis’.
STATISTICAL ANALYSIS
Data were presented as number (percentage) or median (minimum–maximum), as indicated. The ROC curve, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using SPSS version 27.0 (IBM, Armonk, New York, USA) in order to assess diagnostic accuracy of TE against histopathology. Area under the ROC (AUROC) of 1.0 represents an excellent test; however, AUROC of ≤0.5 indicates a worthless test.17 Categorical variables were compared between groups using Fisher’s exact test. P<0.05 was considered statistically significant.
RESULTS
The authors identified 35 patients with a history of MTX use who underwent FibroScan to screen for possible MTX-induced liver injury. One patient had a prior history of alcohol use disorder that was in remission at the time of FibroScan. Of this sample, 23 (66%) were female. The median age was 63 years (range: 32–74 years) and median BMI was 32.1 (range: 20.6–46.3). MTX was used by patients to treat rheumatologic or dermatologic diseases in 26 patients (74%), and haematologic diseases in nine patients (26%). Rheumatologic/dermatologic indications included rheumatoid arthritis in 15 patients (58%), psoriasis or psoriatic arthritis in seven (27%), scleroderma in two patients (8%), and one case for each of atopic dermatitis and inclusion body myositis. Haematologic indications were lymphoma/leukaemia in four patients (45%), myelodysplastic syndrome in three patients (33%), and myelofibrosis in two (22%). LSM ranged from 3.8–74.8 kPa with a median of 10.8. CAP ranged from 118–400 dB/m. Twenty-seven of the patients (77%) underwent a liver biopsy. Of those, nine patients (33%) had advanced liver fibrosis on biopsy. Of the eight patients who did not undergo biopsy, three had a clinical diagnosis of cirrhosis. Thus, 12 of the 35 patients (34%) were considered to have advanced liver fibrosis based on clinical and histopathological data. Table 1 shows a summary of the demographic, clinical, TE, and histopathologic results of the study cohort.
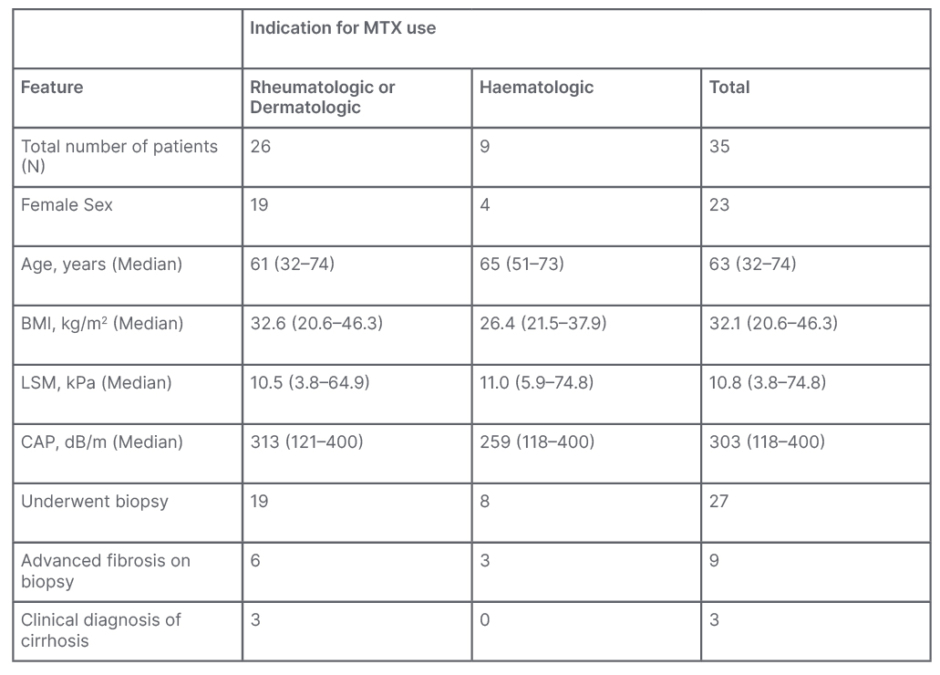
Table 1: Demographic, clinical, elastographic, and histopathological data of the authors’ population.
CAP: controlled attenuation parameter; LSM: liver stiffness measurement; MTX: methotrexate.
A total of 14 patients had LSM values of less than 10.0 kPa on TE. Of those patients, only one was found to have advanced liver disease confirmed by biopsy. Figure 1 shows the scatterplot of LSM values for patients with confirmed non-advanced and advanced liver fibrosis, respectively. Sensitivity of FibroScan for ruling out advanced fibrosis when kPa is <10.0 was calculated to be 92% and NPV was 93%. These data can be found in Table 2. Table 3 shows that for LSM cut-off value of 15.0 or greater, 10 total patients were identified. Of those patients, seven were found to have advanced fibrosis according to METAVIR classification. Specificity of FibroScan for ruling in advanced fibrosis when kPa is ≥15.0 was calculated to be 87%, while PPV was 80%. Evaluation of these data using a ROC curve showed an area under the curve of 0.80 (Figure 2).
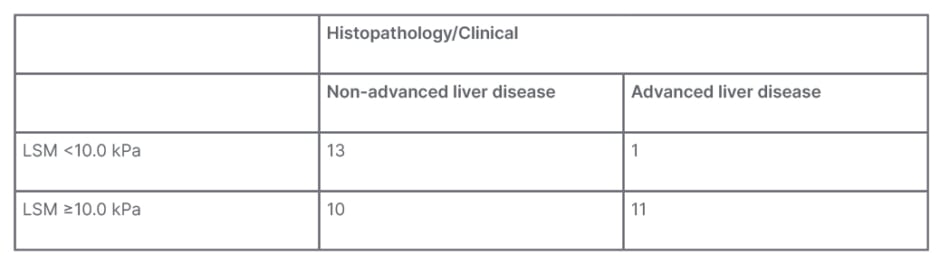
Table 2: Clinical/histopathological advanced liver disease and liver stiffness measurement of 10 kPa.
LSM: liver stiffness measurement.
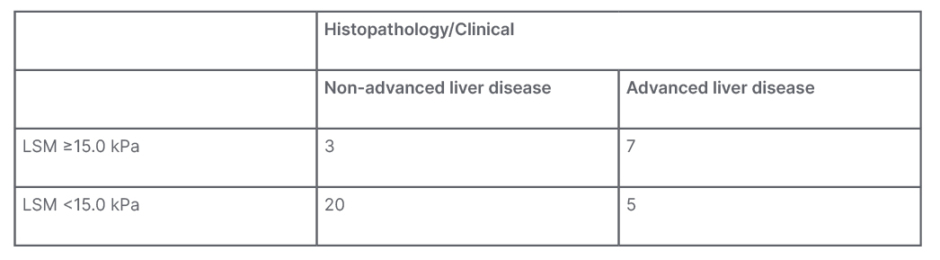
Table 3: Clinical/histopathological advanced liver disease and liver stiffness measurement of 15 kPa.
LSM: liver stiffness measurement.
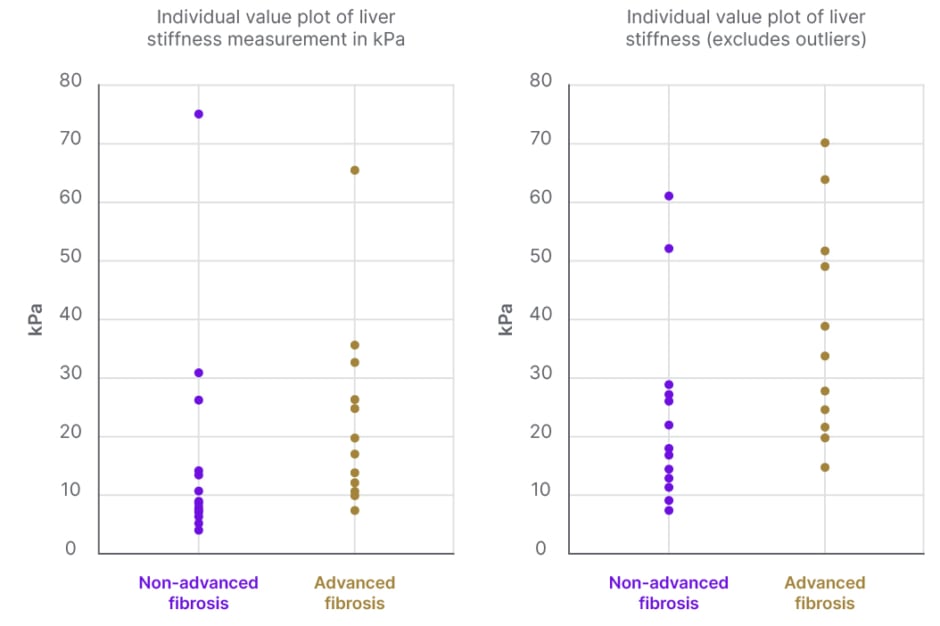
Figure 1: Scatter-plot diagram showing the distribution of liver stiffness values (kPa) between advanced and non-advanced fibrosis confirmed clinically or by histopathology.
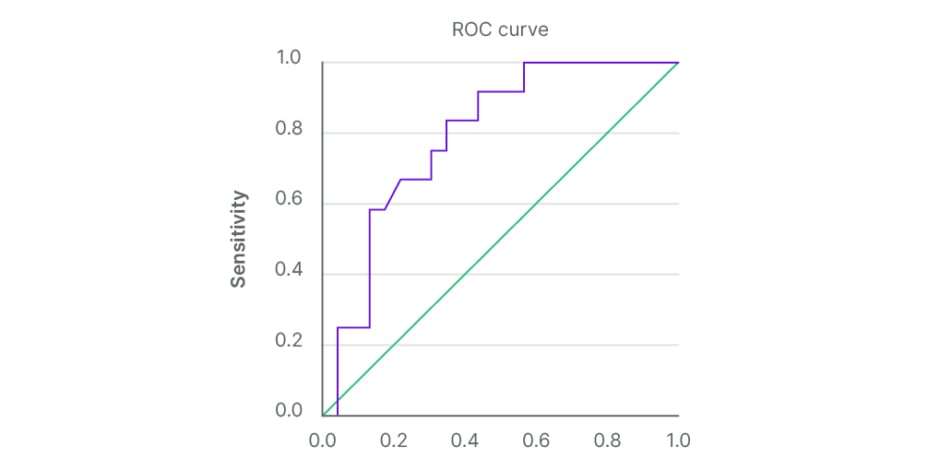
Figure 2: Receiver operating characteristic curve for prediction of advanced liver fibrosis based on kPa measurements.
Diagonal segments are produced by ties.
ROC: receiver operating characteristic.
Treatment with MTX was continued after evaluation for MTX-induced liver injury in six out of 35 patients. Of these, five had reassuring biopsy results (score of F0, F1, and F2) and one had a low LSM value of 4.6 kPa. Elastography was performed in two out of six of the patients continued on MTX, and showed stable kPa. MTX was initially stopped then restarted in three patients, after repeat TE showed improvement of LSM values. A follow-up FibroScan was performed in eight out of 35 patients and showed stable LSM values in six patients, and worsening LSM in two patients, even after stopping the MTX. Six out of the 35 were deceased, none of them developed hepatocellular carcinoma, and none received liver transplant.
DISCUSSION
The use of MTX is associated with increased risk of liver fibrosis and cirrhosis.18,19 Given the inherent risk of liver biopsy with associated increased morbidity and mortality,20,21 non-invasive techniques have been used to assess for liver fibrosis. TE has been adopted as a routine investigation technique for monitoring of liver injury in patients treated with MTX.3 Elastography can help measure liver stiffness through LSM and predict the degree of steatosis through CAP. Prior studies have shown mixed results in the sensitivity and specificity of FibroScan to detect advanced fibrosis, compared to histopathology. The authors’ study was conducted to add to the available data on the performance of FibroScan in this population.
Although low LSM cut-off values have been previously adopted in literature,22,23 other guidelines recommend the use of higher cut-offs, since elastography has high sensitivity and specificity when used to detect cirrhosis rather than lower stages of liver fibrosis.3,24,25 Hence, the authors used kPa cut-off of <10 kPa to rule out advanced chronic liver disease, kPa between 10–15 to suggest possible advanced disease, and LSM >15 kPa to be highly suggestive of advanced liver disease. In this cohort, the median LSM was 10.8 kPa and 33% of the cohort had advanced liver disease based on clinical and histopathological evidence. This percentage of advanced disease is in accordance with results from a recent review showing that 0–33% of patients on MTX can develop significant hepatic fibrosis.3
The authors’ study showed a sensitivity of 92% and NPV of 93% in ruling out advanced liver fibrosis with kPa <10.0. Furthermore, the sensitivity and NPV were 100% for a kPa of <7.0. The high sensitivity in ruling out advanced fibrosis indicates FibroScan is a useful screening test for patients at risk of MTX-induced fibrosis. This was supported by a smaller study by Bray et al.,26 which used a kPa cut-off of 7.1, for which the sensitivity was 100% in detecting liver fibrosis.26 However, two studies by Berends et al.27 of 24 patients, and another by Rongngern et al.23 of 45 patients, used a cutoff of 7.1 kPa, and found sensitivity to be 50% for detecting ≥F2 liver fibrosis.22,27 Importantly, the authors’ study measured the ability of FibroScan to detect ≥F3 liver fibrosis. Thus, the higher sensitivity than the latter two studies is likely, due in part to the fact FibroScan can more readily detect a more advanced form of liver disease.3,25 In addition, it is important to mention that the latter studies were performed on patients with psoriasis who commonly have higher incidence of metabolic syndrome, and obesity is a common cause for failure of elastography.28
Using a higher LSM cut-off point of kPa ≥15, the authors’ specificity was calculated at 87% and PPV at 80%. The AUROC was 0.80 (95% confidence interval). These findings indicate a reasonable ability of FibroScan to rule in advanced fibrosis in our population, though they were not as significant as the sensitivity and NPV. These results are in accordance with Berends et al.,27 who showed that TE has 88% specificity for detection of ≥F2. On the contrary, a recent prospective cross-sectional study demonstrated a low predictive ability of elastography to identify fibrosis with an AUROC of 0.41.22
The authors’ literature review showed that guidelines regarding the use of MTX following the identification of MTX-induced liver injury are scarce. In their cohort, they were able to restart MTX in a total of nine out of 35 patients. All nine patients had low kPa on elastography and/or F0, F1, or F2 fibrosis stages on histopathology. Results for follow-up FibroScans were available in two cases showing stable kPa after restarting MTX. Larger and prospective studies are needed to help establish standardised guidelines for continuation of MTX in such settings.
The authors’ study has several limitations, including being retrospective and having a small sample size. While the authors did exclude patients with other more common aetiologies of liver fibrosis (viral hepatitis, active alcohol use, diagnosis of non-alcoholic fatty liver disease), any patient with another covert aetiology of fibrosis would introduce confounding into the population. Additional limitations included lack of biopsy in eight of the patients, and lack of available data on cumulative doses of MTX.
In conclusion, the authors’ retrospective study shows that FibroScan has high sensitivity and specificity for kPa 10 and 15, respectively, for detecting advanced liver fibrosis in patients on MTX. Higher powered studies, and ideally prospective studies, are needed to validate these findings in the context of mixed results among the available small studies. Verification of the utility of FibroScan can increase the confidence in which we interpret non-invasive findings for this population and potentially avoid unnecessary liver biopsies.







