Abstract
The conventional conception of the therapy of heart failure (HF) with reduced ejection fraction has been recently modified by adding sodium-glucose co-transporter-2 (SGLT2) inhibitors to the combination consisting of beta blockers, mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors, with the aim of improving clinical outcomes. It remains unclear whether other sub-populations of patients with HF, having either HF with preserved ejection fraction (HFpEF) or HF with mildly reduced ejection fraction, are relevant candidates for the effective therapeutic intervention that includes SGLT2 inhibitors.
The purpose of the narrative review is to elucidate plausible perspectives for the clinical implementation of SGLT2 inhibitors into optimal medical therapy in patients with HFpEF. The authors searched the bibliographic databases (Embase, Medline, and the Web of Science) and the Cochrane Central to find English-written publications satisfying the purpose of this study. The authors included eight studies and two meta-analyses that have been reported as completed and found that there were high heterogeneous data regarding the fact that SGLT2 inhibitors had strict resemblance in their efficacy among patients with HFpEF with and without Type 2 diabetes. Due to the use of unpublished data and findings from the trials ended early, there is a lack of upper left ventricular ejection fraction threshold levels to identify inclusion criteria and no agreement in heart failure with reduced ejection fraction determination. However, the results of the meta-analysis, especially come from subgroups’ analysis, appeared to be relevantly optimistic for use of SGLT2 inhibitors in HFpEF therapy.
INTRODUCTION
Despite a moderate trend of the incidence of new cases of heart failure (HF) and HF with reduced ejection fraction (HFrEF) to decline, mainly in developed countries, the estimated absolute number of prevalent HF with preserved ejection fraction (HFpEF) seems to steadily increase in both developed and developing countries as the result of ageing and the comorbid conditions of the population.1,2 Rising costs for medical care and the implementation of several modern technological innovations into routine clinical practice sufficiently increased the burden of HF.3,4 The American Heart Association (AHA) has reported that the real total of direct medical costs as a result of HF is said to increase from 21 billion USD in 2012 to 53 billion USD in 2030 in the USA.5 Moreover, these expenditures seem to be projected without double counting the direct costs that are attributed to several comorbid conditions related to HF development.5 In fact, guideline-directed medical therapy in patients with HFrEF is based on using angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors, which has been shown to be suboptimal due to low target drug dose achievement and respectively high discontinuation rate over 12 months.6
In addition, event rates for HF hospitalisation or premature death remain unacceptably high and strongly associated with a disproportional growth of expenditures on medical services.6-9 Obviously, traditional treatment of patients with HF requires improvement. Moving away from the old conception (mentioned above) to new four pillars treatment scheme including angiotensin receptor-neprilysin inhibitors, beta blockers, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors (particularly dapagliflozin and empagliflozin), the authors have received new data from several large clinical trials and meta-analyses, which have demonstrated remarkable improvement of prognosis and noticeable attenuation of cost-efficacy in patients with HFrEF compared with standard therapy.10-12 However, it remains unclear whether other subpopulations of patients with HF, having either HFpEF and HF with mildly reduced ejection fraction, are relevant candidates for the effective therapeutic intervention that includes SGLT2 inhibitors. The purpose of the review is to elucidate the plausible implementation of SGLT2 inhibitors into optimal medical therapy in patients with HFpEF.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS AND CARDIOVASCULAR BENEFITS IN PATIENTS WITH AND WITHOUT DIABETES
SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, luseogliflozin, and ertugliflozin) combined with the SGLT1/2 inhibitor sotagliflozin are modern innovative drug classes, which were initially designed as antidiabetic agents.13 They have demonstrated beneficial effects on fasting and postprandial hyperglycaemia through decreasing glucose reabsorption as a result of a blockage of SGLT2 proteins, which are abundantly expressed in the proximal convoluted tubule of the kidney and ensure re-absorption of about 90% of all glucose.14 Indeed, these agents being administered as a monotherapy and in combination with other glucose-lowering therapies, including metformin and insulin, which have shown significant reductions in HbA1c and fasting glucose without an adverse impact on cardiovascular (CV) safety compared with placebo.15
In addition, SGLT2 inhibitors exerted a sufficiently lower risk of hypoglycaemia compared with sulphonylureas and similarly low risk as metformin, pioglitazone, or sitagliptin.15 In patients with Type 2 diabetes (T2D), the SGLT2 inhibitor canagliflozin provided a remarkable reduction of body weight, which contributed to a decrease in systolic blood pressure.16 However, previous clinical studies have yielded strong evidence regarding the tissue-protective activity of SGLT2 inhibitors.17 Along with it, SGLT2 inhibitors were found to be able to attenuate CV and renal outcomes in long-term studies in patients with T2D with known CV diseases, including myocardial infarction, chronic kidney disease, and HF, as well as in individuals with traditional CV risk factors.18-20
The meta-analysis of 40 clinical trials by Benham et al.21 revealed that SGLT2 inhibitors led to a much more pronounced reduction of total CV events in patients with T2D compared with placebo, but there was no significant association between the risk of CV events and decrease in blood pressure. Another meta-analysis of 27 studies (N=7,363) has shown that the administration of SGLT2 inhibitors was associated with lowered HbA1c coupled with blood pressure, body weight, and albuminuria in patients with T2D and chronic kidney disease.22 Therefore, SGLT2 inhibitors exhibited a significant reduction in the risk of CV death, non-fatal myocardial infarction or non-fatal stroke, and HF without an effect on all-cause death.22 Consequently, SGLT2 inhibitors have been initially approved by highly reputed medical associations for the therapy of T2D and then they went on to turn in HFrEF regardless of the presence of T2D.
The first SGLT2 inhibitor that received approval by the U.S. Food and Drug Administration (FDA) for treatment of HFrEF, with the aim of reducing the risk of CV death and hospitalisation in patients regardless of the presence of T2D, was dapagliflozin. Shortly after, the FDA approved another SGLT2 inhibitor, empagliflozin, for the same indication.23 Current clinical guidelines recommend both dapagliflozin and empagliflozin for the therapy of HFrEF, but not HFpEF due to limiting solid evidence.24-26 However, exact molecular mechanisms that are involved in the beneficial impact of SGLT2 inhibitors on CV outcomes in HF continue to remain to be uncertain.27
PLAUSIBLE MECHANISMS OF ACTIONS OF SODIUM-GLUCOSE CO-TRANSPORTER-2 INHIBITORS
Although a broad range of pleiotropic effects of SGLT2 inhibitors have been previously found and thoroughly investigated, the interrelation between direct hypoglycaemic effects and indirect pleiotropic effects requires additional explanation.28 Figure 1 illustrates a large variety of opinions about the role of several molecular pathways contributing to the effects of SGLT2 inhibitors.
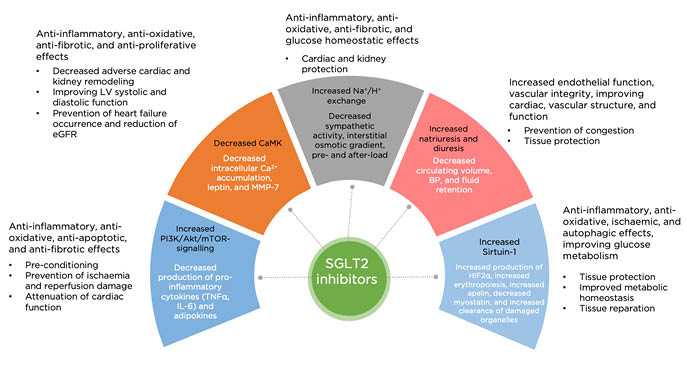
Figure 1: Plausible molecular mechanisms contributed to the beneficial effects of SGLT2 inhibitors in patients with heart failure.
Akt: serine/threonine-specific protein kinase; BP: blood pressure; Ca2+: calcium; CaMK: Ca2+: calmodulin-dependent protein kinase; eGFR: estimated glomerular filtration rate; HIF: hypoxia inducible factor; LV: left ventricular; PI3K: phosphatidylinositol 3-kinase; MMP: matrix metalloproteinase; mTOR: mammalian target of rapamycin; SGLT2; sodium-glucose co-transporter-2.
The most simple assumption that is considered to be realistic is a decrease in sustained systolic and diastolic blood pressure, resulting in natriuresis and sympathetic tone, which can translate into improvement of CV prognosis and slowing kidney disease progression. Perhaps, bodyweight reduction can be a potential mechanism in the alleviation of CV risk.29 Apart from this, it has been hypothesised that SGLT2 inhibitors can be involved in the regulation of the sodium–hydrogen exchange in the heart and kidney leading to both cardiac and renal protection. Through their stimulating effect on diuresis and natriuresis, these agents can decrease the interstitial osmotic gradient, pre- and after-load, and thereby potentially improve vascular structure and function.30 Acting as stimulators of erythropoiesis due to the ‘mimicking’ effect of systemic hypoxia on the kidney, SGLT2 inhibitors seem to be powerful triggers for non-specific tissue protection.31,32 In addition, they may modulate the production of a wide spectrum of adipokines (leptin, visfatin, adiponectin), myokines (apelin, irisin), and inflammatory cytokines (TNF-α, IL-6) acting through the sirtuin-related signalling pathway.33 This signalling pathway is also responsible for the turnover of myocardial energy homeostasis from glucose utilisation to oxidation of other substrates, such as ketone bodies, free fatty acids, and branched-chain amino acids, which appear to be a powerful modulator for mitochondrial function playing a pivotal role in pre-conditioning and oxidative stress.33 Finally, the sirtuin-1 pathway seems to be a central player in SGLT2-related regulation of reducing cardiac cells necrosis and cardiac or kidney fibrosis.34
BENEFITS OF THE SODIUM-GLUCOSE CO-TRANSPORTER-2 INHIBITORS IN HEART FAILURE WITH PRESERVED EJECTION FRACTION
There is data about 19 clinical trials that have been initially designed with the aim of elucidating the effect of SGLT2 inhibitors in patients with HFpEF, with and without T2D, on clinical outcomes and the surrogate points (mainly the levels of cardiac biomarkers), but only eight from these had a completed status along with available results to evaluate (Table 1). In addition, nine randomised clinical trials have been reported as permanently completed, but the study design provided for the possibility to enrol patients with T2D for whom HF was not determined as inclusion criteria (EMPA-REG, CREDENCE, DECLARE-TIMI-58). However, post-hoc analysis was frequently performed with the aim of elucidating the impact of SGLT2 inhibitors on either cardiac biomarkers or HF-related outcomes.
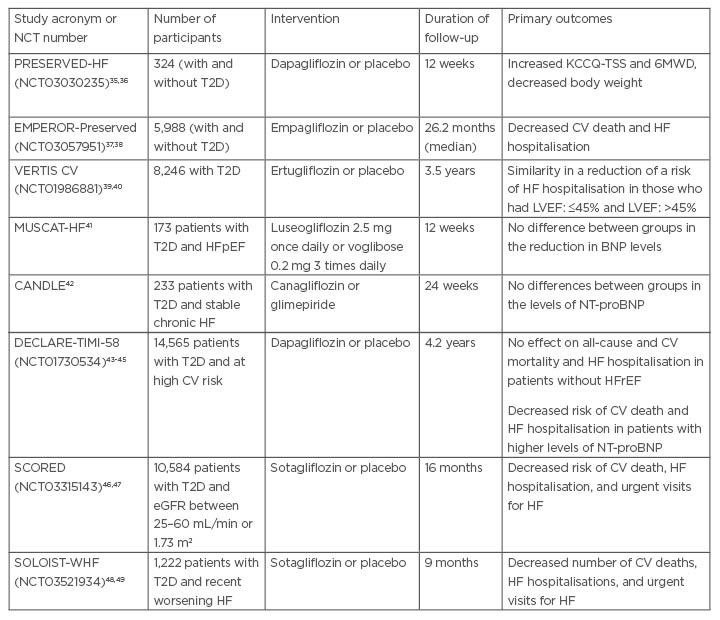
Table 1: Completed randomised clinical trials dedicated the impact of sodium-glucose co-transporter-2 inhibitors on clinical status of patients with heart failure with preserved ejection fraction.
BNP: brain natriuretic peptide; CV: cardiovascular; eGFR: estimated glomerular filtration rate; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; KCCQ-TSS: Kansas-City Cardiomyopathy Questionnaire-Total Symptom Score; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro–B-type natriuretic peptide; NCT: National Clinical Trial; T2D: Type 2 diabetes; 6MWD: 6-minute walk distance.
Nassif et al.35 evaluated 324 patients with HFpEF (left ventricular ejection fraction [LVEF]: >40%) who were randomly included in the groups of dapagliflozin (10 mg daily) or placebo.35 The authors reported that dapagliflozin noticeably improved Kansas-City Cardiomyopathy Questionnaire (KCCQ)-Clinical Summary Score, six-minute walk distance, and reduced body weight compared with placebo over 12 weeks, whereas there were no significant differences between both groups in systolic blood pressure and the levels of natriuretic peptides and HbA1c.35,36
The EMPEROR-Preserved trial has been enrolled 5,988 patients with class II–IV HFpEF (LVEF: >40%) to receive empagliflozin 10 mg/day or placebo added on optimal therapy for HF and comorbidities.37,38 Eligible patients had either the levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP) of ≥300 pg/mL without atrial fibrillation or >900 pg/mL with atrial fibrillation due to established structural heart disease within 6 months prior to study entry or hospitalisation within 12 months before being included in the trail.37 Therefore, 49% of the patients had T2D. An estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2 was found in 50% of eligible patients and atrial fibrillation was diagnosed in 51% of all recruited individuals.37 The primary outcome (CV death or HF hospitalisation) occurred in 13.8% and 17.1% for empagliflozin group and placebo group, respectively (hazard ratio [HR]: 0.79; 95% confidence interval [CI]: 0.69–0.90; p<0.001).37 The authors emphasised that the benefit was noticed to be similar in patients regardless of the presence of T2D, but patients with HFpEF and LVEF of ≥60% exerted less advantage in composite clinical outcome. However, amongst secondary outcomes, total hospitalisations, and change in mean eGFR slope per year were found to be significantly reduced in empagliflozin group when compared with placebo group (p<0.001 for all cases), whereas improvement in all-cause mortality, composite renal outcome, new onset T2D among patients with pre-diabetes were not remarkably changed during the study.37 In addition, the meaningful improvement in KCCQ-Clinical Summary Score was more likely to notice in empagliflozin versus placebo.37 Thus, the results of the study have yielded that superiority of empagliflozin to placebo was associated with a reduction in HF hospitalisations and quality of life, but not with all-cause and CV mortality and renal outcomes.37
VERTIS CV was specially designed to elucidate whether ertugliflozin reduces HF hospitalisation and CV death in patients with T2D and atherosclerotic CV disease.39,40 Unlike in EMPEROR-Preserved trial, this study has been included 8,246 patients with T2D, 1,958 of which had a history of HF (HFrEF: n=959; HFpEF: n=999). All patients were allocated into two groups (ertugliflozin 5 mg or 15 mg daily [n=5,499] or placebo [n=2,747]) and followed for a mean of 3.5 years.39 The results of the study have ascertained that ertugliflozin reduced the risk of first HF hospitalisation and the total number of HF admission to hospitals with strict similarity in patients with HFpEF and HFrEF. In addition, the authors noticed a remarkable decrease in the combined outcome (total HF hospitalisation or CV death) in eligible patients.39
Novel SGLT2 inhibitor luseogliflozin has been investigated in a small study called MUSCAT-HF, in which 173 patients with T2D and HFpEF (LVEF: ≥45%; BNP: ≥35 pg/mL) were included.40 All eligible patients were allocated to receive luseogliflozin (n=83) 2.5 mg once a day or voglibose (n=82) in 0.6 mg daily for 12 weeks. The authors reported that there was no remarkable difference between groups in the reduction in the plasma levels of BNP.41
Tanaka et al.42 elucidated the impact of canagliflozin on the changes in circulation on the levels of cardiac biomarkers such as NT-proBNP in patients with T2D and chronic HF. They included 233 patients having a mean LVEF value of 57.6% (standard deviation: 14.6%), so 71% of eligible patients had been diagnosed with HFpEF (LVEF: ≥50%).42 All patients were randomised to receive canagliflozin 100 mg or glimepiride (initial daily dose was 0.5 mg with follow-up titration) and followed for 24 weeks. Unfortunately, the levels of NT-proBNP were not found to show a sufficient reduction during the observation period in both groups. Moreover, the authors did not find significant differences between groups in this parameter at the end of the study.42
In the DECLARE-TIMI-58 trial, dapagliflozin (10 mg daily) exerted a significant risk reduction of the composite outcome (CV death or HF hospitalisation) compared with placebo in patients with T2D, while the study was not designed as HF trial.43-45 Indeed, only 1,464 patients (10.1%) from the 14,565 who were selected had a history of HF, mainly HFrEF, which was defined as LVEF: <45%, but not as LVEF: <40%.43 Therefore, 1,316 (7.7%) had HF without known reduced LVEF.40 Zelniker et al.43 measured baseline NT-proBNP levels in patients who were enrolled in the study and found that the mean values were 75 pg/mL (interquartile range: 35–165 pg/mL]. Importantly that dapagliflozin reduced the risk of the composite outcome regardless of NT-proBNP levels, although the effect of the agent was found to show greater absolute risk reductions in patients with T2D having higher baseline NT-proBNP concentrations compared with those who had lower ones.40 Kato et al.44 reported that dapagliflozin remarkably reduced all-cause mortality in patients with HFrEF (HR: 0.59; 95% CI: 0.40–0.88), but not in those who had no HFrEF.44
SCORED was a multicentre double-blind randomised clinical trial that enrolled patients with T2D and chronic kidney disease and then allocated to receive sotagliflozin (n=5,292) or placebo (n=5,292).46,47 The median of the observation was 16 months; however, the trial was ended early due to loss of funding and might have affected the results. To note, the majority of eligible patients had HFpEF defined as LVEF: ≥50%. The authors found that sotagliflozin was superior to placebo in a reduction of CV death, HF hospitalisation, and urgent visits for HF, but was associated with numerous adverse events, such as diarrhoea, genital mycotic infections, volume depletion, and diabetic ketoacidosis.46
The SOLOIST-WHF trial depicts to elucidate the impact of sotagliflozin on CV death, HF hospitalisations, and urgent visits for HF in patients with T2D and recent worsening HF.48,49 A total of 1,222 patients with T2D with either HFpEF (LVEF: ≥50%) or HFrEF (<50%) were randomised to receive sotagliflozin (n=608) or placebo (n=614) after reaching haemodynamic stability and then were followed for 9 months.48 Participants had elevated BNP levels (≥150 pg/mL for patients without atrial fibrillation and ≥600 pg/mL for those who had atrial fibrillation). The results have yielded much more pronounced reduction of primary endpoints in the sotagliflozin group compared with the placebo group (HR: 0.67; 95% CI: 0.52–0.85; p<0.001).48 All these data reflect a favourable trend to lower NT-proBNP/BNP levels and/or quality of life in patients with HFpEF treated with SGLT2 inhibitors. Along with it, the benefit of the agents in keeping with CV outcomes and HF-related complications remained uncertain. Perhaps it relates to short follow-ups, unknown HF phenotypes and upper LVEF limit for inclusion at the baseline, and high variety in age and gender in different studies.
A recent meta-analysis of nine randomised clinical trials (n=19,741) by Singh and Singh50 yielded the significant risk reduction in composite endpoint ([CV death and/or HF hospitalisation] HR: 0.74; 95% CI: 0.69–0.79; p<0.001), with CV death (HR: 0.86; 95% CI: 0.78-0.95; p=0.003) and HF hospitalisations (HR: 0.68; 95% CI: 0.62-0.74; p<0.001) with SGLT2 inhibitors in patients with chronic HF. Analysis of the subgroup did not show a benefit in the composite of CV death or HF hospitalisation in patients with HFrEF or HFpEF, so these findings require more investigations in the future.
Another meta-analysis of eight large clinical trials by Lu et al.51 confirmed these conclusions (mentioned above) and demonstrated that SGLT2 inhibitors noticeably decreased the risk of composite end-point (CV death or HF hospitalisation) by 23% (HR: 0.77; 95% CI: 0.72–0.82), HF hospitalisations by 32% (HR: 0.68; 95% CI: 0.62–0.75), and CV death by 15% (HR: 0.85; 95% CI: 0.76–0.94) in patients with known HF, regardless of its phenotype. Obviously, the results of both meta-analyses are based on the hypothesis that the proportion of patients having HFpEF in the studies enrolled to the investigations and the qualification of clinical outcomes have been thoroughly possessed; however, innate restrictions such as the use of unpublished data and findings from trials that ended early, a lack of upper LVEF threshold levels to identify inclusion criteria, and no agreement in HFrEF determination are limitations for them.50,51
However, the effect of SGLT2 inhibitors being independent from T2D status is considered to be clearly elucidated in specifically designed trials dedicated to outcomes in patients with known HFpEF. In order to compare the results of these studies and prevent misunderstanding, the universal definition of HFpEF is used to stratify patients at risk and diagnose HF phenotype for all studies that are going to conduct. Whether dapagliflozin and empagliflozin are effective in prevention of CV death or worsening HF in patients with HFpEF, independent of their T2D status, will hopefully be discovered when two new large clinical trials are will be completed in the near future. The DELIVER52 and EMPERIAL-Preserved53 trials are now in progress.
GAPS OF KNOWLEDGE AND FUTURE PERSPECTIVES
Nowadays the definition of HFpEF is an object of scientific discussion and several previous studies that are considered to have been dedicated SGLT2 inhibitors in HF have, in reality, been provided in a wide range of patients who not only had HFpEF but also HF with mildly reduced ejection fraction, unconfirmed HF phenotype, and those with LVEF: ≤60%. There are serious concerns that the data obtained cannot be an attribute of the bias and high variability in the effects. This means, in particular, that it will be difficult to extrapolate data received from placebo-controlled trials into actual clinical practice, even if studies with relatively small sample sizes had been shown positive effects of SGLT2 inhibitors on surrogate endpoints in HFpEF (LVEF: <40%). This is particularly true if some patients with HFrEF might have serious benefits in terms of increasing LVEF until the threshold is over 49%. Although this fact is not considered to be a cause to change a phenotype of HFrEF to HFpEF during SGLT2 inhibition, new values of LVEF should be pondered in case of interpreting the results. The next concern relates to uncertainty in the decision-making of the regulatory authorities in many countries because the prescription of SGLT2 inhibitors according to new indications such as HFpEF is still restricted by them and requires solid approval. In addition, there is no consent on how the metabolic phenotype influences the drugs’ efficacy in HFpEF. Animal studies have clearly revealed that SGLT2 inhibition can attenuate cardiometabolic dysregulation of cardiac function and modify the altered myocardial structure, but there is a serious deficiency in clinical evidence. However, these clinical findings might represent as novel therapeutic targets for the treatment of HFpEF with SGLT2 inhibitors associated with reduced all-cause and CV mortality.
CONCLUSION
Recent clinical studies for SGLT2 inhibitors exhibited heterogeneous data regarding the fact that these agents had a strict resemblance in their efficacy among patients with HFpEF with and without T2D. However, the results of meta-analysis, especially come from subgroup analysis appeared to be relevantly optimistic for use of SGLT2 inhibitors in the therapy of HFpEF, because of a lack of difference in dynamics of cardiac biomarkers amongst patients with HFrEF and HFpEF and there was a steady trend to improve HF hospitalisation. The DELIVER trial and the EMPERIAL programme, including the EMPERIAL-Preserved trial, are addressed to the question whether SGLT2 inhibitors are powerful agents to reduce all-cause and CV mortality in HFpEF.







