Abstract
A spontaneous subscapular hepatic haematoma is a rare condition that has not widely been reported in literature. Subscapular hepatic haematoma has a high mortality rate, especially if the haematoma ruptures, so early diagnosis is imperative. In this case report, the authors present an unusual case of subscapular hepatic haematoma of a female in her 70s, who, in her first few days of admission for the management of acute calculus cholecystitis, developed acute onset right upper quadrant and epigastric pain radiating to her back. Her haemoglobin dropped from 112 g/L to 54 g/L, and her liver function tests and coagulation studies became deranged.
Abdominal and pelvic CT and angiography showed a subscapular liver haematoma without active bleeding. The patient received a blood transfusion and was managed conservatively, with no obvious cause being identified.
Key Points
1. This manuscript describes the presentation of a rare disease process that is not well understood, but has the potential to be fatal.2. The authors discuss the aetiology, presentation, investigation, and management of a patient who developed a spontaneous subcapsular haematoma, as well as the importance of early recognition.
3. Consider spontaneous subcapsular haematoma when assessing a patient with right upper quadrant pain, nausea/vomiting, and hypovolaemic shock, especially in the presence of risk factors such as pregnancy or liver lesions.
INTRODUCTION
A spontaneous subcapsular hepatic haematoma (SHH) is both a rare and potentially life-threatening condition. Although the pathogenesis of SHH is not well understood, it is most primarily attributed to complications of liver lesions or pregnancy.1-3 Presenting clinical signs are not specific, but can include right upper quadrant (RUQ) or epigastric pain, severe shoulder pain, nausea, vomiting, shortness of breath, abdominal distention, and hypovolaemic shock.3,4 Diagnosis can be delayed due to rarity and variability of presentation but is made by imaging, ultrasound, CT, or MRI. Management is either conservative or surgical.3,5
CASE PRESENTATION
A 79-year-old female (patient A) presented to the emergency department with RUQ, back, and flank pain. She denied any history of trauma, including trivial, to the area. Her past medical history included ischaemic heart disease, with a previous coronary artery bypass surgery; congestive cardiac failure; atrial fibrillation; Type 2 diabetes, managed with insulin; gallstones; obesity; osteoarthritis; and osteoporosis. It is important to note that she was not taking an oral anticoagulant despite her diagnosis of atrial fibrillation. This was the result of a risk/benefit decision due to her risk of falls combined with her age and already being on aspirin. On physical examination, she had a tender RUQ, no guarding or peritonism, and present bowel sounds. She was haemodynamically stable: respiratory rate: 18; O2 saturations: 97% on room air; heart rate: 82 bpm; blood pressure: 160/90 mmHg; and a low grade temperature of 37.5–38.0 °C. Her blood sugar level was 10.4 mmol/L.
Her blood tests on admission, including haemoglobin and liver function tests, were largely within normal ranges, and in line with patient A’s baseline (Table 1). Coagulation studies were not completed; this is notable and cannot be explained specifically. It is potentially attributable to an assumption that there was no initial concern of coagulopathy, and that she would likely not be a surgical candidate having been deemed not fit for a cholecystectomy previously in view of her extensive co-morbid state.
Initial blood cultures yielded no growth, and urine culture grew Escherichia coli.
A CT abdomen/pelvis with contrast showed a 17.0 mm calculus impacted in the gallbladder neck, with associated distended gallbladder, but without definite evidence of cholecystitis, pericholecystic fluid, or extrahepatic bile duct dilation. The pancreas was severely atrophic and the spleen unremarkable. There were no urinary tract calculi or obstruction, and marked pelvic floor prolapse shown. A targeted ultrasound of the abdomen showed a 28.0 mm non-mobile gallstone seen at the gallbladder neck. The gallbladder was distended, with diffuse thickening of the gallbladder wall to 5.5 mm. There was no pericholecystic fluid. Features were compatible with acute calculus cholecystitis.
Following surgical review, patient A was admitted under the joint care of the general medical and general surgical teams in view of her complex medical background, and managed conservatively for acute calculus cholecystitis. She was commenced on a low-fat diet, and treated with intravenous (IV) antibiotics as per local guidelines (ceftriaxone and metronidazole), with consideration of a percutaneous cholecystostomy if she were to acutely deteriorate. Subsequently, her RUQ pain and tenderness started to improve, so it was assumed that the antibiotics were working, and the cholecystitis improving.
Three days into her admission, patient A developed acute worsening of the pain in her back and RUQ, which was not responsive to opiates; she had no nausea or vomiting, and her bowels were opening regularly. She described the pain as similar in character to her presenting pain, but with increased intensity. She was tender generally in her abdomen, but particularly in the RUQ. Bowel sounds were present, and her observations were stable. Patient A was reviewed by the surgical team, but it was decided further imaging was not needed at this stage, as there were no signs of peritonitis, and pancreatitis was unlikely as the gallstone was impacted and not obstructed. Instead, it was decided to monitor her blood results and treat symptomatically.
Patient A became progressively more hypotensive over the next 24–48 hours and her pain continued to worsen. Her IV antibiotics were escalated to piperacillin/tazobactam. She initially appeared responsive to IV fluids; however, she became notably pale, with cool peripheries and weak distal pulses. Her full blood count and renal and liver function tests became deranged (Table 1), with her haemoglobin dropping to 54 g/L, and blood film showing rouleaux++, anisocytosis++, polychromasia+, elliptocytes+, and nucleated red blood cell+. Coagulation studies showed an international normalised ratio of 1.5, with a prothrombin time of 17 seconds.
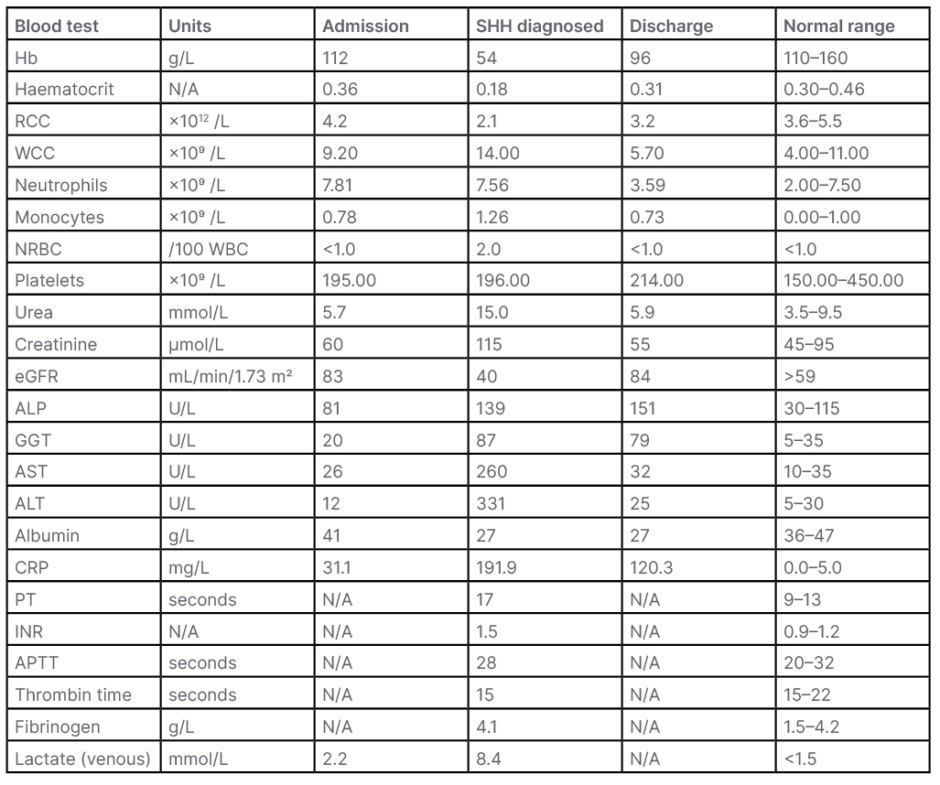
Table 1: The sequence of haematologic, biochemical, and coagulation tests performed prior, at the onset of clinical deterioration, and following recovery.
ALP: alkaline phosphatase; ALT: alanine transaminase; APTT: activated partial thromboplastin time; AST: aspartate transferase; CRP: c-reactive protein; eGFR: estimated glomerular filtration rate; GGT: γ-glutamyltransferase; Hb: haemoglobin; INR: international normalised ratio; N/A: not applicable; NRBC: nucleated red blood cells; PT: prothrombin time; RCC: red cell count; SHH: subcapsular hepatic haematoma; WBC: white blood cell; WCC: white cell count.
The source of blood loss was unclear at first. Patient A was taking no oral anticoagulant, there was no external blood loss, and she denied a history of trauma. She was taking regular aspirin lifelong following her coronary artery bypass surgery, and was prescribed enoxaparin 40 mg subcutaneously once daily since admission for venous thromboembolism (VTE) prophylaxis, having been deemed to be at increased risk of VTE in part due to her age, large body habitus, and significantly reduced mobility associated with hospital admission. A rectal examination
was normal.
A CT of the abdomen and pelvis was repeated, and a CT angiogram performed (Figure 1), which confirmed new changes of haemoperitoneum and a large subcapsular haematoma. Mildly hyperdense fluid could be seen extending from the inferior liver border into the pelvis via the right paracolic gutter. No active bleeding site was identified on the arterial, portal venous, or delayed phase imaging.
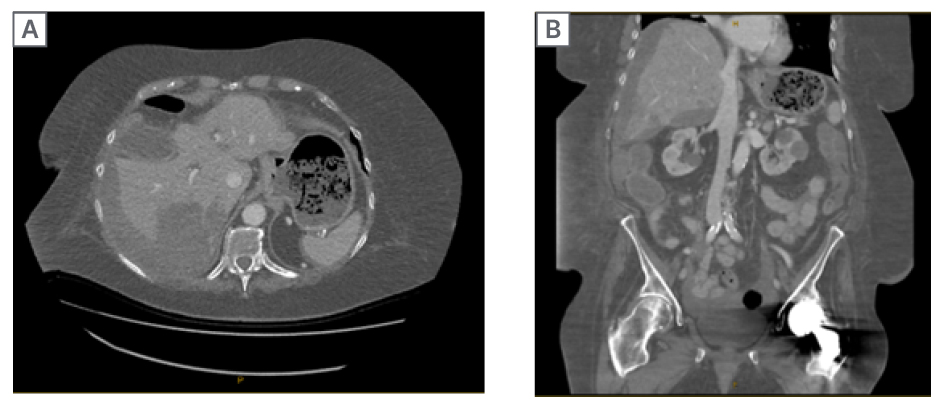
Figure 1: CT angiogram of the abdomen/pelvis.
Axial (A) and coronal (B) views, showing a large subcapsular haematoma.
Patient A was managed acutely with 3 units of red blood cells, and both enoxaparin and aspirin were ceased. She responded well, with her haemoglobin rising to 96 g/L pre-discharge (Table 1). She remained stable, and her liver function tests and coagulation began to improve along with her symptoms clinically.
In view of there being no active bleed on imaging, along with weighing up her cardiac risk factors, she was managed conservatively. She was prescribed morphine through a patient controlled analgesia pump for pain, and she slowly improved. Following family discussions, her ceiling of care was adjusted to ward based. She had an extended stay due to slow recovery and poor mobility, but was eventually discharged around 1 month after admission.
No cause for patient A’s SHH was found, despite extensive testing. COVID-19 PCR was negative, and viral screening was negative for HIV. Cytomegalovirus and Epstein–Barr virus testing were found to be positive only for past infection, hepatitis A IgG was suggestive of previous exposure, and hepatitis B and C screening showed no evidence of infection. Changes in serum free light chains were consistent with ageing/renal impairment, and rheumatoid factor and serum protein electrophoresis were unremarkable. No paraprotein was detected, and antinuclear antibody, glomerular basement membrane antibodies, and Igs yielded no results. An atypical anti-neutrophil cytoplasmic antibody was considered of no significance, and thyroid function was unremarkable. There was no indication of malignancy or any hepatic lesions on imaging.
DISCUSSION
First described in 1844, SHH involves the spontaneous collection of blood between the capsule of Glisson and the liver parenchyma.1,3,4 The pathogenesis is not well described, due to the rare nature of the condition, and there are very few cases available in literature. It commonly affects the right lobe of the liver, and is attributed mostly to complications of pre-eclampsia, eclampsia, and/or haemolysis, elevated liver enzymes, and low platelet count syndrome.1,6 In SHH related to pregnancy and haemolysis, elevated liver enzymes, and low platelet syndrome, it is thought that the deposition of fibrin triggers platelet activation, resulting in thrombi that can occlude capillaries and evolve into hepatic necrosis and haemorrhage.2,3,7
Other causes include malignancy (such as angiosarcoma, haemangioendothelioma, hepatoblastoma, and rhabdoid sarcoma), underlying benign liver lesions (including haemangioma, adenoma, focal nodular hyperplasia, nodular regenerative hyperplasia, biliary cystadenoma, and angiomyolipoma), coagulopathies and vasculitides, and liver trauma and iatrogenic injuries (such as endoscopic retrograde cholangiopancreatography, liver biopsy, biliary surgery). It can also be associated with connective tissue disease, peliosis hepatis, amyloid, systemic lupus erythematosus, and polyarteritis nodosa.1-3,5,6 A diagnosis of SHH is often the result of clinical suspicion in patients with a known underlying condition, such as those discussed above. However, it is not always as simple. Patients often present with non-specific symptoms, commonly acute onset RUQ, epigastric, or shoulder pain. Nausea and vomiting can also occur due to hepatic parenchyma distension.3,4,7
It can, therefore, be misinterpreted to be an alternative intra-abdominal pathology, which present similarly, such as cholecystitis, or in pregnancy, the RUQ pain seen in pre-eclampsia.7 In the case of patient A, the potential delay in diagnosis was the result of the radiologically confirmed presence of cholecystitis. Patients with SHH can become haemodynamically unstable, presenting in hypovolaemic shock.3,4 This can be symptomatic of a ruptured haematoma, most commonly in the setting of trauma, and has a 75% mortality rate.2 Close monitoring is, therefore, needed.7
Prior to developing SHH, Patient A was prescribed both aspirin and a prophylactic dose of enoxaparin. This was in keeping with the regional VTE guidelines for the prescription of prophylactic dose anticoagulation. The use of an antiplatelet, such as aspirin in the case of patient A, is deemed only a relative contraindication to prophylactic enoxaparin with risk/benefits to be considered.
Following VTE risk assessment, patient A was deemed as moderate- to high-risk for developing an inpatient VTE, with risk factors including an expected inpatient for more than 2 days, along with risk factors including obesity, age over 60, and significantly reduced mobility associated with hospital admission.8 It was deemed appropriate to continue both antiplatelet and anticoagulant therapy.
SHH is not an expected complication of either aspirin or prophylactic enoxaparin. The overwhelming available literature regarding bleeding as a complication involves therapeutic dose enoxaparin. Moreover, major haematoma remains an uncommon side effect, and related bleeding is usually minor, with only an estimated 0.5–4.0% of patients prescribed therapeutic enoxaparin found to experience major bleeding.9 There have been some reports of rectus sheath, retroperitoneal, and pelvic haematomas from therapeutic enoxaparin.10,11 To the authors’ knowledge, there have been no spontaneous subcapsular haematomas attributed to prophylactic dose enoxaparin. There have been cases of spontaneous haematoma reported in literature involving patients taking dual antiplatelet therapy (aspirin and clopidogrel).12,13 One case report was found describing a spontaneous perirenal haematoma found following low dose aspirin.14 The risk of clinically relevant bleeding is, however, higher when receiving both and should be highlighted.
In regard to the diagnoses of SHH, blood laboratory results can show anaemia and deranged hepatic and coagulation profiles, but final diagnosis is through imaging (ultrasound, CT, or MRI).5
Depending on the setting, a bedside ultrasound is often the most rapid investigation, with focused abdominal ultrasound for trauma examination undertaken in those who are haemodynamically unstable. CT and MRI are both highly sensitive for SHH; however, CT is often more easily accessible and, therefore, more appropriate in a deteriorating patient, as was the case with patient A.7 The high protein levels present in acute haematomas will appear hyperdense on imaging.1
Liver injuries can be graded by the American Association for the Surgery of Trauma (AAST) liver injury scale classifying hepatic injuries into categories I–VI.15 Two-thirds of liver injuries are between Grades I–III as was the case with patient A.7 Although there is no set protocol for the management of SHH, and each patient is managed on a case-by-case basis, the AAST acts as guidance.15
In haemodynamically stable patients, unless the haemorrhage is ongoing, conservative management is often favoured (including fluids and blood products), as in the case we have reported. However, in unstable patients, a hepatic haematoma is considered an acute surgical emergency. Surgical options to achieve homeostasis can be a challenge, and include hepatic artery embolisation, packing, drainage, hepatic artery ligation, hepatic resection of affected areas, or even transplantation. Hepatectomy may be needed for bleeding due to neoplasms.1,3,5,7,15-18
Although complete resolution of a SHH can take a significant length of time, as was the case with patient A, if the patient’s condition and the haematoma remain stable, discharge and outpatient monitoring can be appropriate.7
CONCLUSION
In conclusion, SHH is an uncommon diagnosis, especially in the absence of risk factors such as pregnancy. Presentation can be variable, and patients can deteriorate rapidly. SHH can be considered when assessing any patient presenting with RUQ pain, nausea, vomiting, or hypovolaemic shock. Management options vary, and involvement of both physicians and surgeons is important. As the authors report above, treating conservatively can yield a positive result, and the authors hope that this case report will raise awareness of the diagnosis, which has the potential to be fatal. Use of low dose anticoagulants being attributed to major life-threatening bleeding is not well reported and was not considered to be a cause in this presentation, although it is noteworthy to mention as more research may be conducted regarding this in the future.







