Abstract
Background: Infection, especially Staphylococcus aureus bacteraemia (SAB), increases morbidity and mortality in patients with chronic kidney disease (CKD), particularly those who are dialysis-dependent. This study describes the clinical features of SAB amongst patients with CKD.
Method: The authors planned a retrospective observational study of adult patients with CKD and SAB from January 2021–June 2022. Microbiology laboratory data and hospital medical records were reviewed. All detailed clinical data, including baseline characteristics, source of infection, management, methicillin susceptibility of S. aureus isolates, and outcome, were collected. The association between baseline characteristics, source of infection, management, and outcome of patients, was examined.
Results: The authors included 49 patients in their study. Methicillin-resistant S. aureus was more prevalent (35 out of 49; 71.4%) than methicillin-sensitive S. aureus (14 out of 49; 28.6%). Central venous catheter was the most common source of infection (38 out of 49; 77.6%). Most patients recovered (26 out of 49; 53.1%), while 12 (24.5%) were referred to other hospitals. Four patients expired. Methicillin-resistant S. aureus bacteraemia had higher mortality than methicillin-sensitive S. aureus bacteraemia. Central line-associated bloodstream infections showed higher mortality than other sources of infection, although due to a small sample size, this difference could not be proven statistically. SAB showed significant association with patient outcomes. The central venous catheter could not be removed in three of four expired patients (p=0.018).
Conclusion: SAB is a serious but preventable nosocomial infection in patients with CKD who are dependent on dialysis. Strict infection prevention measures are needed to prevent hospital-acquired infections in these patients.
Key Points
1. Staphylococcus aureus bacteraemia is the most common cause of infection in patients with chronic kidney disease who are dialysis-dependent. This paper adds to the existing data from Pakistan.2. This article describes the association between baseline characteristics, the source of infection, management of, and outcomes of patients with S. aureus bacteraemia. Methicillin-resistant S. aureus bacteraemia had higher mortality than methicillin-sensitive S. aureus bacteraemia. Central line-associated bloodstream infection showed higher mortality than other sources of infection.
3. S. aureus bacteraemia is a serious but preventable nosocomial infection in patients with CKD who are dialysis-dependent. Strict infection prevention measures are needed to prevent hospital-acquired infections in these patients.
INTRODUCTION
Patients with chronic kidney disease (CKD), especially those on chronic haemodialysis (HD), have a higher incidence of nosocomial infection than non-dialysis-dependent patients who are hospitalised, due to repeated exposure to the healthcare environment. Mortality from all causes in patients on dialysis treatment is 6.5–7.9 times higher than that of the general population. In patients ≥65 years of age, mortality from infection is two times higher than that in a population of the same age with other comorbidities, such as diabetes, neoplasms, or congestive heart failure.1-3 The risk and incidence of infection increases with catheter use for vascular access.4,5 Staphylococcus aureus bacteraemia (SAB) is a leading cause of infection in these patients.6,7
S. aureus (SA) is a part of normal skin flora, and is the most common cause of bacteraemia in patients who are dependent on HD. Repeated hospital exposure, intravascular catheter use, invasive procedures, skin barrier disruption, and impaired renal function increase the risk of SAB in these patients.8 SAB has many complications, as well as increased morbidity and mortality.9 It is associated with severe, often fatal complications, such as endocarditis, osteomyelitis, septic arthritis, empyema thoracis, pneumonia, and meningitis, despite relevant antibiotic treatment.10 Methicillin-resistant S. aureus (MRSA) bacteraemia has a higher risk of mortality than methicillin-sensitive S. aureus (MSSA) bacteraemia.11
The authors’ institution, The Kidney Centre Postgraduate Training Institute, Karachi, Pakistan, is a not-for-profit specialised hospital, which provides care to patients with kidney disease. It is mainly a renal disease centre, with a large dialysis unit. Around 200 dialysis sessions take place per day at the institute. Multiple patients are awaiting renal transplant, and they need access for dialysis, usually through an arteriovenous fistula (AVF), which takes at least a few weeks to mature. During this period, dialysis is carried out via central venous catheters (CVC).12 Central line-associated blood stream infection (CLABSI), especially with SA, is a very commonly encountered hospital-acquired infection in these patients. Previous studies from Pakistan show that there is a high burden of SA infection, especially with MRSA, in this patient group.13,14 There are limited reports on the clinical features and outcomes of SAB in patients with CKD in Pakistan. More local data are required to see the current burden of infection, especially with a preventable cause like SAB, in this patient group; this will highlight the importance of preventing infections, especially SAB. This study describes the clinical features of SAB among patients with CKD at a tertiary care renal centre.
METHODS
The authors carried out a retrospective observational study of patients with SAB from January 2021–June 2022. All patients with SAB who were registered at The Kidney Centre Postgraduate Training Institute were included in the study; this included all admitted patients, those routinely receiving dialysis at the institution, and those who presented to the emergency room. The authors excluded all non-registered patients, and all cases with bacteraemia with other organisms. Exemption was obtained from the ethical review committee of the institute (ref. no. 152-IDC-102022[EXEMPTION]).
Microbiology laboratory data and hospital medical records were reviewed. All detailed clinical data, including baseline characteristics, source of infection, management, methicillin susceptibility of SA isolates, and outcome, were collected. Association between baseline characteristics, source of infection, management, and outcome of patients was examined.
STATISTICAL ANALYSIS
The data were analysed using IBM SPSS Statistics version 26.0 (IBM, Armonk, New York, USA). Mean with standard deviation was evaluated for continuous variables, while frequency with percentage was calculated for categorical data. Association among the following variables was observed with the patient outcome, using the chi-squared test: methicillin susceptibility of SA; source of infection; type and site of line; central line management; and treatment. A p value of ≤0.05 was considered significant.
RESULTS
The authors included 49 patients in this study, with a female to male ratio of 1.00:1.58, and a mean age of 51.0±18.4 years (minimum: 9 years; maximum: 91 years). Hypertension (36 out of 49; 61.2%) and diabetes (27 out of 49; 55.1%) were the most prevalent comorbidities. Diabetic nephropathy was the major known cause of CKD (10 out of 49; 20.4%; Table 1A).
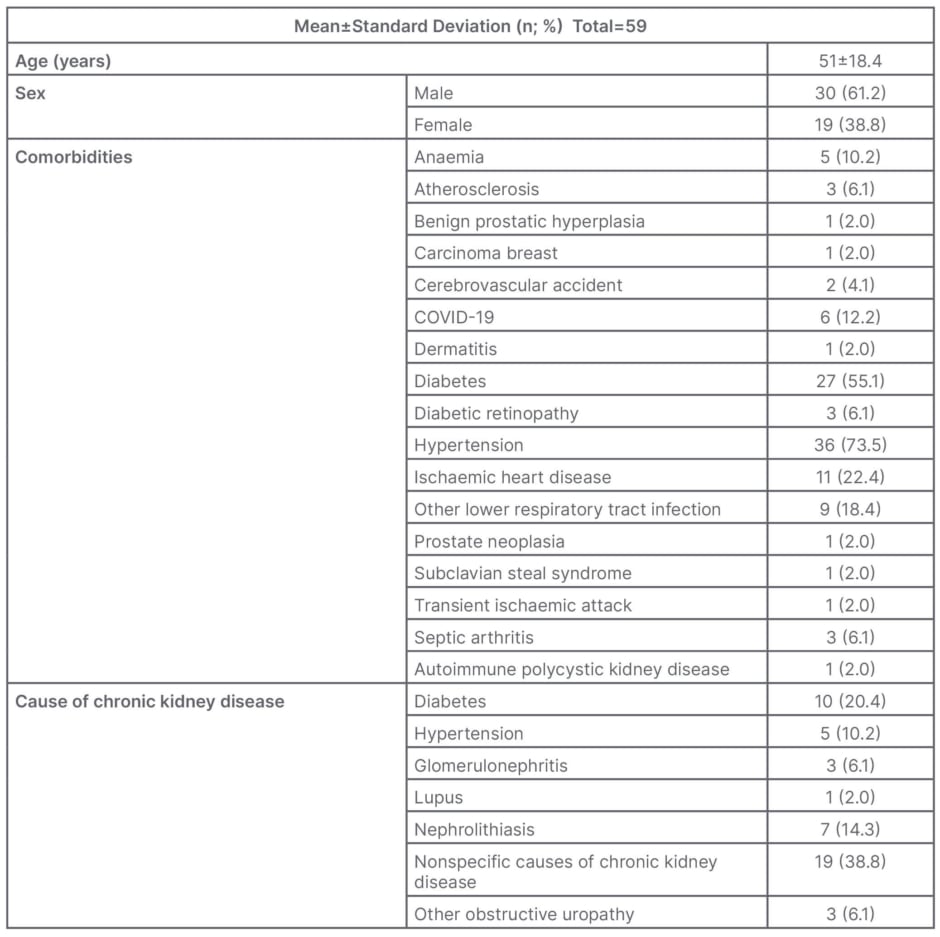
Table 1A: Baseline characteristics of patients.
MRSA was more prevalent (35 out of 49; 71.4%) than MSSA (14 out of 49; 28.6%). CVC was the most common source of infection (38 out of 49; 77.6%). Most of the CVCs were temporary (22 out of 49; 44.9%). Internal jugular (28 out of 49; 57.1%) was the most common line insertion site. In most cases, vancomycin was the drug of choice (34 out of 49; 69.4%; Table 1B).
Most patients recovered (26 out of 49; 53.1%), while 12 (24.5%) were referred to other hospitals (Figure 1).
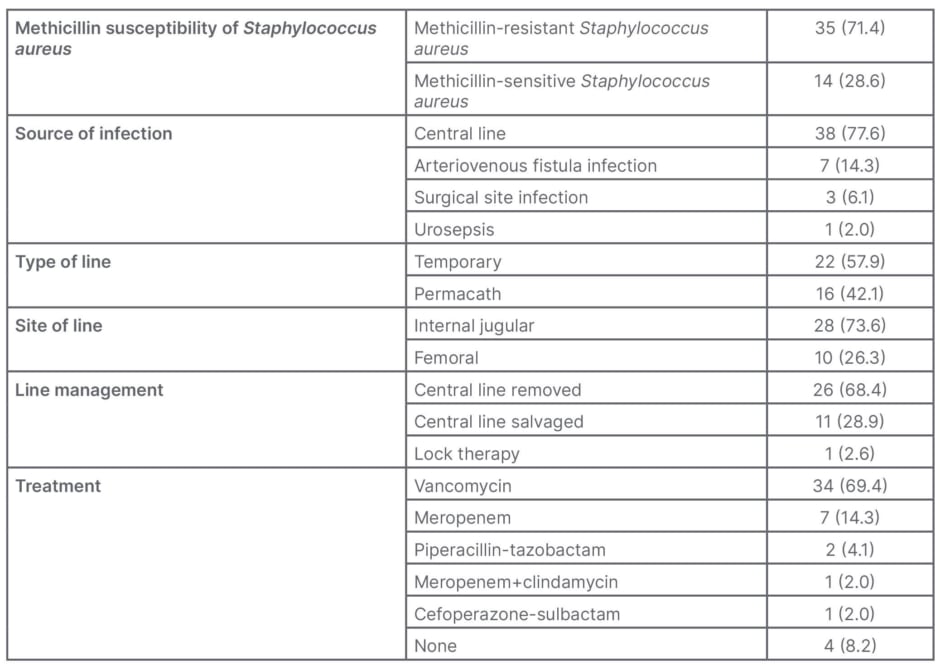
Table 1B: Clinical parameters of patients (n; %).
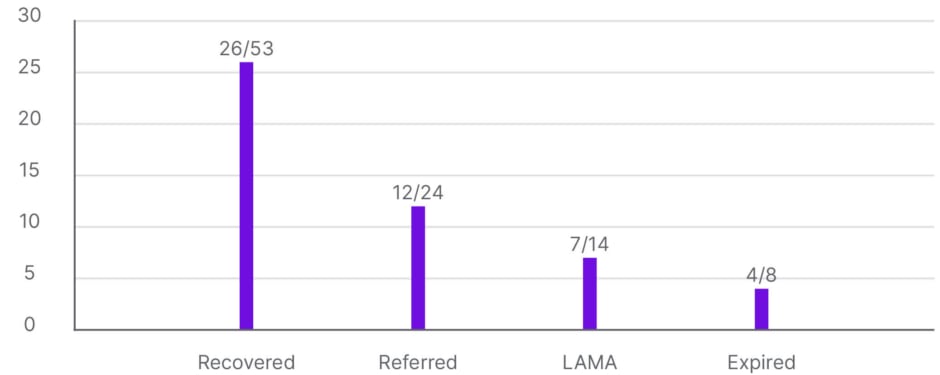
Figure 1: Patient outcomes.
LAMA: left against medical advice.
Three (75.0%) of four expired patients were male, although this was statistically insignificant (p=0.064). All four patients who died had hypertension, and three (75.0%) were also diabetic, but this was also statistically insignificant. Since the authors’ institute is a specialised hospital for renal diseases, and does not offer treatment for COVID-19 infection, almost all patients who tested positive were referred to COVID-19 care facilities. One patient who tested positive expired (Table 2A).
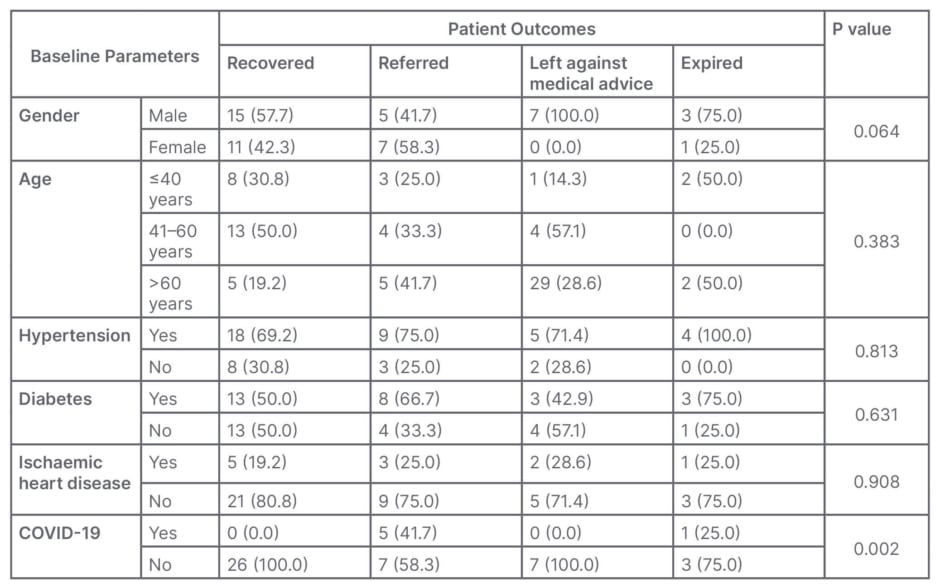
Table 2A: Association between baseline characteristics and patient outcomes (n; %).
MRSA bacteraemia had a higher mortality rate than MSSA bacteraemia. Similarly, CLABSI showed higher mortality than the other sources of infection, although due to a small sample size, this difference could not be proven statistically (Table 2B).
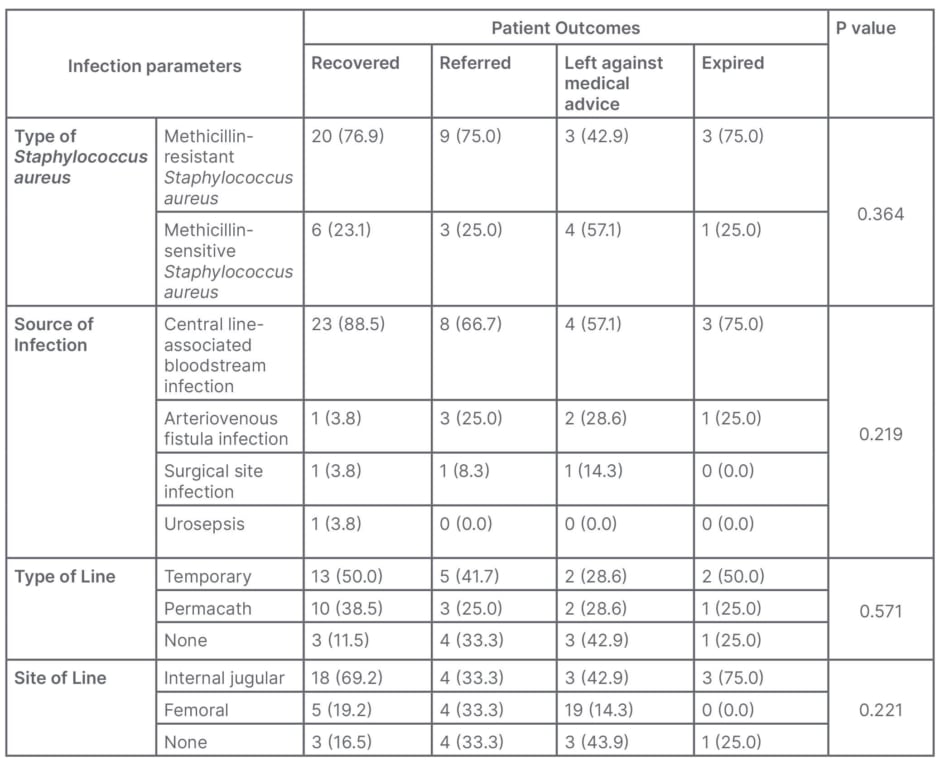
Table 2B: Association between infection variables and outcome of the patients (n; %).
The management of SAB showed a significant association with patient outcomes. The CVC could not be removed in three of four expired patients due to non-availability of venous access for dialysis (p=0.018; Table 2C).
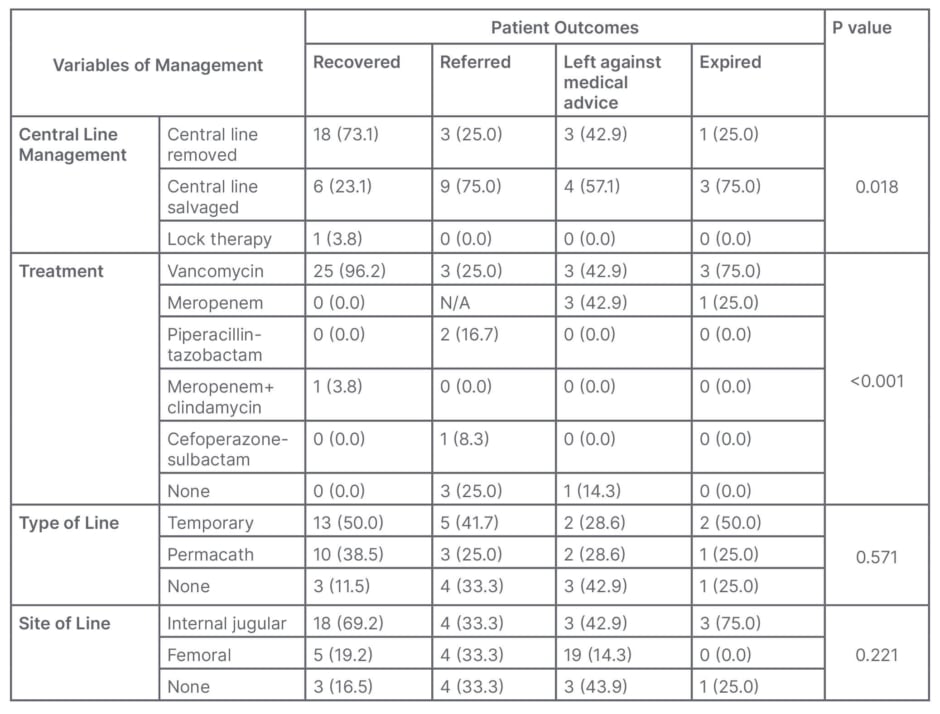
Table 2C: Association between variables of management and patient outcomes (n; %).
N/A: not applicable.
DISCUSSION
SAB is a leading cause of infection in patients with CKD who are dialysis-dependent, due to vascular access and repeated hospital exposures. According to a report by the Centers for Disease Control and Prevention (CDC), patients who were dialysis-dependent were 100 times more likely to have an SAB than adults not on dialysis.15 This can be prevented by appropriate infection prevention and control strategies.
Though a lot of international data are available, as a first step, it is important to know the burden of this infection in the authors’ patient population. They retrospectively observed the clinical features of patients with CKD and SAB at a tertiary specialised hospital for renal care. CVC was the major source of SAB in their patients, and MRSA dominated MSSA. Most of the patients were empirically started on meropenem and vancomycin, with the continuation of vancomycin and discontinuation of meropenem after the finalisation of cultures. Timely source removal and appropriate antibiotic use were associated with survival.
SAB causes higher morbidity and mortality rates in patients with CKD, especially those who are dialysis-dependent, as compared to patients without CKD. Cheon et al.16 compared the clinical outcomes associated with SAB in patients with and without CKD. High Sequential Organ Failure Assessment (SOFA) score and proportion of administration of inappropriate antibiotics were associated with CKD.16 In a cross-sectional study from Rawalpindi, Pakistan, Jamil et al.17 determined the prevalence of bacteraemia in patients with CKD, particularly those on dialysis, and those with renal transplants. They reported the frequency of SAB among patients with CKD and on haemodialysis to be 15%.17 MRSA prevalence was detected to be 2%.
Haemodialysis vascular access, particularly when using a CVC, increases the risk of SAB and sepsis compared to AVF.12,18,19 Long-term CVC is associated with serious complications, such as CLABSI.20 In a previous study from the authors’ institute, Qureshi et al.12 concluded that out of a total of 429 non-tunneled catheters, 50 (17%) were removed due to CLABSI. In these findings, MRSA was the most common organism responsible.12 The authors’ findings are consistent with this. CLABSI (38 out of 49; 77.6%) was the major source of infection in the studied cohort, with a predominance of MRSA (35 out of 49; 71.4%).
In a similar study from Pakistan, Shahnila et al.13 described the clinical presentation of SAB, and outcomes from a tertiary care hospital. SAB increases morbidity and mortality in patients with CKD. Haemodialysis and CVCs were the major risk factors of SAB. Similar to the authors’ findings, out of 92 patients, 81 (88%) had MRSA, and 11 (12%) MSSA. MRSA bacteraemia presented with more complications.13
MRSA bacteraemia possesses a higher risk of mortality compared to MSSA bacteraemia.11 Of the authors’ four patients who expired, three had MRSA bacteraemia. Multiple studies from Pakistan have previously reported the increasing frequency of emerging resistance to methicillin in SA.14,21,22 In a descriptive study in Rawalpindi, Ali et al.14 recovered 100 (42.01%) MRSA samples from 238 SA isolates, in different clinical samples.
SAB has increased the mortality rate in patients with COVID-19.23 The authors had six patients with both SAB and COVID-19. Five of these were referred to COVID-19 care facilities. One patient with MRSA bacteraemia and COVID-19 expired, as a result of an AVF infection. Despite timely treatment with vancomycin, the patient succumbed.
The clinical presentation of SAB might differ from previous patients with SAB. Changes in the characteristics of the patient population, as well as clinical and molecular epidemiology of SAB in patients receiving maintenance HD, and in the clones causing SAB, may contribute to increased morbidity and mortality.24,25
The small sample size is the major limitation of the authors’ study, and means that statistical significance could not be proven in many of their findings. The authors were unable to follow their patients for SAB complications. Despite this, their research supports that SAB led to increased morbidity and mortality in their institution’s patients with CKD who are dialysis-dependent.
Preventive measures need to be prioritised. Catheter locks should be considered when the CVC needs to be salvaged. There needs to be a focus on improving infection prevention protocols. An appropriate staff-to-patient ratio with strict infection prevention measures is needed to prevent SAB in patients undergoing dialysis. MRSA decolonisation can be an option to decrease the chances of SAB in those who are colonised.19 Better antimicrobial surveillance is needed to decrease emerging antimicrobial resistance. To prevent SAB, the first step can be detecting CKD in its early stages to prevent or delay the need for dialysis, including methods to manage diabetes and high blood pressure, as well as providing education on treatment options among all patients, particularly those at greatest risk, to slow the progression of CKD.15
CONCLUSION
SAB is a serious but preventable nosocomial infection in patients with CKD who are dialysis-dependent. Strict infection prevention measures are needed to prevent hospital-acquired infections in these patients.







