Abstract
Introduction: Hypertriglyceridaemia (HTG) is common and often precipitates into acute pancreatitis. Early diagnosis of HTG pancreatitis (HTGP) is essential for appropriate management to avoid recurrence of pancreatitis. Plasmapheresis was suggested as treatment modality to decline triglyceride levels, especially in critical patients with multiorgan failure. Few randomised studies are recorded regarding the value of plasmapheresis over classical therapy.
Objective: To evaluate the value of plasmapheresis in patients with HTGP with worrisome signs as fever, tachycardia, high inflammatory markers, and pancreatitis.
Methods: Clinical course and laboratory markers status after total plasma exchange (TPE) for HTG that is not responding to initial, traditional therapy by insulin infusion was reported.
Results: The authors’ patient had an initial triglyceride level of 30 mmol/L, with a worsening clinical condition and acute pancreatitis. After TPE, there was a significant decline in their triglyceride serum levels (53%) after the first session, leading to marvellous recovery.
Conclusion: The authors suggest treatment with TPE for systemic inflammation and HTGP-induced multiorgan failure. However, further research is necessary.
INTRODUCTION
Hypertriglyceridaemia pancreatitis (HGTP) accounts for 1–35% of acute pancreatitis (AP) cases.1 Acute pancreatitis is an acute inflammatory process of the pancreas. Hypertriglyceridaemia (HTG) is considered the third most common aetiology of AP, following gallstones and alcohol. It is responsible for 1–4% of AP cases and it seems to be more serious than AP caused by other aetiologies.2
The possibility of AP progressively increases when triglyceride levels are >500 mg/dL (5.6 mmol/L) and with levels over 1,000 mg/dL (11.3 mmol/L).3 Management of patients includes supportive treatment for AP and reducing triglyceride levels to less than 500 mg/dL (5.6 mmol/L), with the goal of preventing necrotising pancreatitis and organ failure.4 The approach to initial therapy depends on the severity and scoring of AP and the presence of worrisome clinical signs.5 In patients with severe HTG, it may be beneficial to lower the triglyceride (TG) levels without delay. Plasmapheresis has been shown in some cases to be effective in reducing the TG levels in patients with severe HTG.6 This can be achieved by the rapid removal of TGs from plasma, leading to the reduction of free fatty acids, which can cause further damage to the pancreas.7 Here, the authors present a case of severe HTG with AP, for which the trial of therapeutic plasma exchange modality was reported.
CASE REPORT
A 24-year-old female with no significant medical history nor history of alcohol intake presented at the authors’ hospital with a complaint of a pain in her abdomen (pain scale: 8) and after vomiting for 2 days. They were diagnosed before referral as HTG, which was complicated by AP. They were transferred to the authors’ facility for other intervention for HTG as they were not improving on insulin infusion after 48 hours.
On physical examination, the patient was well-oriented (Glasgow Coma Scale [GCS]: 15/15) and their blood pressure was within normal range; however, they had a high-grade fever, tachycardia, and tachypnoea. Their BMI was 20 kg/m2. An abdominal examination showed diffuse abdominal tenderness but no guarding or rebound tenderness, while an abdominal ultrasound showed an enlarged liver and the bulky hypoechoic pancreas of AP. A laboratory investigation showed evidence of AP due to severe HTG as the patient had an elevated lipase value of 889 units/L and TG level of 2,654.87 mg/dL (30 mmol/L). There were no previous interventions that were relevant to the patient’s condition, nor genetic history of familial HTG. An abdominal CT scan showed pancreatic oedema, suggesting AP. There was a small focal hypodense area at the lower pole of the spleen and right hepatic lobe (segments V and VI) due to infarction, mild pelvic ascites, and mild hepatomegaly.
On the first day of hospital admission, the patient was treated with aggressive hydration with lactated Ringer’s solution; however, due to their worsened clinical condition and marked elevation of TG (30 mmol/L) with fever and leucocytosis, an alternative treatment was discussed with the patient on the second day of hospital admission. The patient consented to therapeutic plasma exchange as a therapeutic intervention.
The patient underwent a total plasma exchange (TPE). During each TPE, 1.5% plasma volume was exchanged and replaced with a 5.0% albumin, which added to a normal saline solution alternate with fresh frozen plasma. Anticoagulation during TPE was achieved with a low-molecular-weight heparin. A review of relevant literature showed many small studies that highlighted the usefulness of early plasmapheresis in this scenario.
A femoral venous catheter was inserted and TPE started within 24 hours of admission. Following the first plasma exchange, the patient’s TGs decreased by 53% to 16 mmol/L (Figure 1).
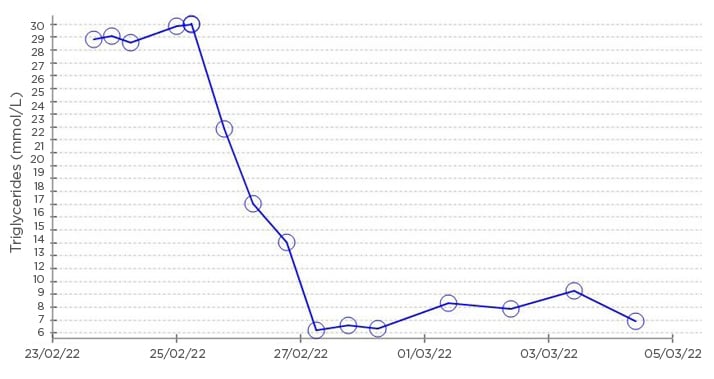
Figure 1: Graphical representation of the biochemical trend over hospital admission, showing triglyceride level.
On the third day of hospital admission, the second session of TPE was completed and decreased TG to 5.65 mmol/L. The patient’s condition improved significantly over the following day: the abdominal pain decreased, and they started feeding orally. A threshold was set for apheresis if TGs were found to be >5.6 mmol/L and rising progressively; however, further sessions were not required.
On the sixth day of hospital admission, the patient started a fibrate therapy and omega-3 fatty acids. A decline in TGs was recorded during their hospital stay. A follow-up CT of the abdomen showed enhancing pseudo-capsulated peripancreatic fluid (12×5 cm), which was in the process of forming into a pseudo cyst. The patient was discharged on the eighth day, with a TG stationary level of 5.8 mmol/L and asymptomatic. The patient followed up on the phone and was doing fine.
DISCUSSION
TG level values of >1,000 mg/dL (11.3 mmol/L) occur in fewer than 1 in 5,000 people.8 Elevated TGs have been reported in patients with underlying genetic predisposition to abnormal lipoprotein metabolism such as familial combined hyperlipidaemia, familial HTG, or other rare abnormalities, precipitating by secondary factors such as excessive alcohol ingestion, insulin resistance, diabetes, nephrotic or metabolic syndrome, and drugs. Uncommonly, a patient with familial HTG with no secondary precipitating factors may also be seen in clinical practice.9
The authors could not find any secondary factors in their patient’s history, so it prompted clinical suspicion for a genetic predisposition. Unfortunately, the authors could not complete genetic study.
Pancreatitis occurs as the result of analysis of TGs into fatty acids by pancreatic enzyme lipases, which leak out of the acinar cells in the vascular bed of the pancreas. This is the probable reason for the accumulation of free fatty acids at high concentrations. Free fatty acids are toxic and they can destroy the acinar cells and capillary endothelium.10 Furthermore, the marked elevation of chylomicrons increase the blood viscosity in the veins and the impaired pancreatic blood flow leads to ischaemia and acidosis in the pancreas.11 Free fatty acids in acidosis activate trypsinogen and initiate acute oedema and necrosis of pancreases.12 The severity of pancreatitis in patients with HTG is the effect of the inflammatory process caused by pancreatitis combined with lipotoxicity from TG hydrolysis.
Severe HTG and high lipase levels lead to high fatty acid levels and can be complicated by systemic inflammation and multiorgan failure from AP.10 The initial treatment of a patient consists of supportive therapy, including fluid resuscitation and pain control that is followed by intravenous (IV) insulin as an infusion therapy, which was found effective in patients in some cases with marked HTGP.13-15
Therapeutic plasmapheresis was suggested and utilised as a possible therapy for HTG.6,16 Evidence to support the value of plasmapheresis in patients with HTGP is from limited small observational studies; large randomised trials are absent.6 One session of plasmapheresis was recorded to decrease TG levels by 50–80%;16 therefore, plasmapheresis may be used in patients with HTGP with alarming parameters, including hypocalcaemia and evidence of systemic inflammation, which involves two or more of the following symptoms: temperature: >38.5 °C; heart rate: >90 beats/min; respiratory rate: >20 breaths/min or partial pressure of carbon dioxide: <32 mmHg; white blood cell count: >12,000 cells/mL or <4,000 cells/mL.6
The authors’ case had fever 39 oC; a tachycardia heart rate of 140 beats/min; leucocytosis; neutrophilia; high inflammatory markers; mild hypocalcaemia; and a high serum lipase that was more than double the normal reference range, which indicated severe pancreatitis. Therefore, the authors started the patient on early TPE. In the authors’ case, the decline in TG level was recorded at 53% following the first session, which suggests that plasmapheresis can be a helpful tool to rapidly and significantly reduce TG levels. Although there are no guidelines for management of HTG-AP, there is a literature review that showed that decreasing TG levels to <500 mg/dL (5.6 mmol/L) can prevent the evolvement of acute pancreatitis.17
The beneficial effects of plasmapheresis are the removal of TGs, active enzymes, and inflammatory plasma mediators, as well as the supplementation of free fatty acids, apolipoproteins, and lipoprotein lipase from a healthy donor plasma. However, accessibility issues in many centres and its high cost limits the use of plasmapheresis. The most recently established guidelines prepared by the American Society for Apheresis (ASFA) notes that HTG-induced AP as a Category III indication (in the case of disorders where the optimum role of apheresis is not established, individualised decision is necessary).18 Fibrates, niacin, and omega-3 fatty acids are the cornerstone of pharmacological therapy.17 Medical therapy, dietary modification, and dietitian counselling are for maintaining goal TG levels.19
CONCLUSION
HTG-induced AP treatment involves the restriction of oral intake; IV hydration; administration of pain medication; an insulin infusion with glucose treatment; and possible TPE. A lipid-free diet and drugs (fenofibrate, gemfibrozil, and omega-3 fatty acids) are utilised to reduce HTG. TPE could be a beneficial treatment modality for patients with HTG-induced AP. Appropriate candidates for apheresis could be patients with severe pancreatitis, who continue to have TG levels of >1,000 mg/dL after the 48 hours of IV insulin.
Further studies that compare the apheresis plus conservative treatment and only conservative treatment in patients with HTG-induced AP patients are required.








