Abstract
Achalasia cardia is the best characterised oesophageal motility disorder. It is characterised by progressive ganglion cell degeneration in the oesophageal myenteric plexus, which results in impaired lower oesophageal sphincter (LES) relaxation upon swallowing and aperistalsis in the distal smooth muscle segment of the oesophagus. The usual presenting features are dysphagia to both liquids and solids from onset, regurgitation of undigested food, retrosternal pain, heartburn, and weight loss. Initial investigations include upper gastrointestinal (GI) endoscopy and timed barium oesophagogram, whereas high resolution manometry is diagnostic. Therapy in achalasia cardia is directed towards biochemical or mechanical reduction in LES pressures. If candidates are fit for surgery, pneumatic dilatation, peroral endoscopic myotomy, and laparoscopic Heller’s myotomy are the mainstays of therapy that act by mechanical disruption of LES. On the other hand, botulinum toxin and pharmacotherapy (nitrates and calcium channel blockers) act by biochemical reduction of LES and are reserved for surgically unfit patients with limited life expectancy because of their short-lived efficacy. Oesophagectomy is reserved for treating refractory longstanding cases, who have previously failed multiple therapies.
INTRODUCTION
Achalasia cardia is a rare oesophageal motility disorder caused by autoimmune neurodegeneration of the oesophageal myenteric plexus.1 Although rare, it is the most common and best characterised oesophageal motility disorder. The primary distinction from other motility disorders (e.g., Jackhammer oesophagus and distal oesophageal spasm) is the failure of lower oesophageal sphincter (LES) relaxation in achalasia. Therefore, most of the therapies are directed towards reduction in LES pressures. Manometrically, achalasia cardia can be divided into three subtypes that aid treatment decision-making and hence have prognostic significance.2,3 There has been renewed interest in this motility disorder in the past few years with the advent of third space endoscopy, such as peroral endoscopic myotomy (POEM), which has revolutionised the endoscopic management of achalasia.4 In this review, the authors discuss the epidemiology, pathogenesis, diagnosis, and treatment of achalasia cardiac.
Epidemiology
Achalasia is equally common in both sexes. Most commonly diagnosed between 40 and 60 years of age, achalasia can present in any age group.3 Most of the epidemiological data is derived from retrospective studies as population-based studies are scarce because of the rarity of achalasia.5 Because of its rarity and chronicity, the prevalence is much higher than the incidence of achalasia. According to the Dutch healthcare insurance data from 2018, the incidence and prevalence of achalasia were 2.2 per 100,000 population per year and 15.3 per 100,000 population, respectively.5 Similarly, Asian data from Korea showed incidence and prevalence of 0.4 per 100,000 population per year and 6.3 per 100,000 population, respectively, in 2014.6According to these studies, the incidence of achalasia is increasing.3
Aetiopathogenesis
Autoimmune progressive degeneration of ganglion cells in the oesophageal myenteric plexus in genetically susceptible individuals (human leukocyte antigen [HLA]-DQ variants DQA1*0103 and DQB1*0603) is the most plausible pathogenetic event leading to achalasia, according to current available evidence.7 There is a preferential loss of inhibitory nitrinergic neurons, which secrete nitric oxide and vasoactive intestinal peptide, and variable a loss of excitatory cholinergic neurons, which secrete acetylcholine, in oesophageal smooth muscles, leading to incomplete LES relaxation and failure of peristalsis.8 The triggering factor for ganglion cell loss is presumed to be latent/chronic viral infections such as herpes simplex virus 1.9 Autoimmune degeneration is mediated by both cytotoxic T cells (cell-mediated immunity) and antibodies to enteric neurons and complement activation (humoral immunity). Achalasia cardia is associated with other neurodegenerative diseases like Parkinson’s disease, as evidenced by the presence of Lewy bodies in ganglion cells.10 Achalasia, like oesophageal dysmotility, can be caused by ganglion cell degeneration by Trypanosoma cruzi, which causes Chagas disease (endemic in South America).11 The combination of achalasia, alacrima, and adrenal insufficiency is known as Allgrove syndrome, or triple A syndrome, which is a rare autosomal recessive disorder.12
DIAGNOSIS
History and Clinical Examination
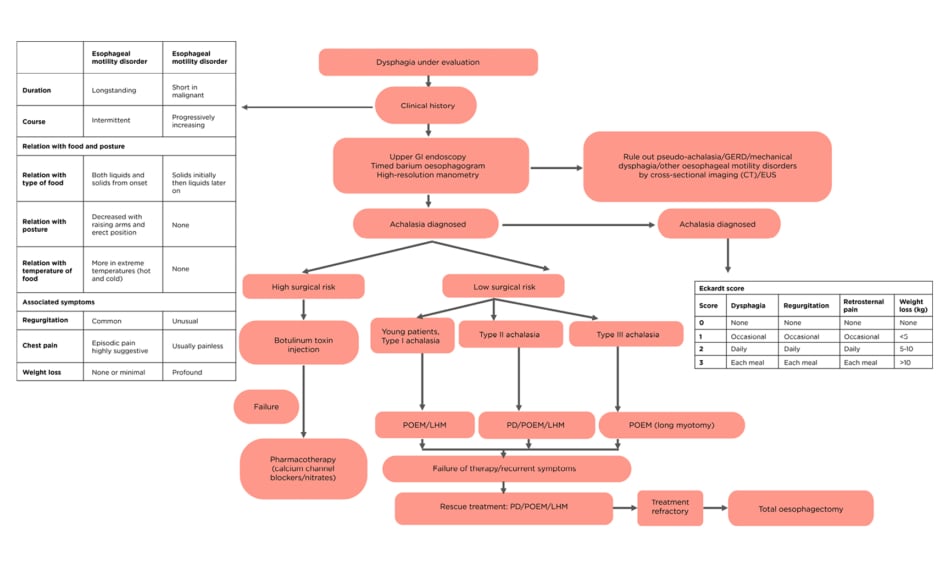
Dysphagia to both solids and liquids from the onset (occurs in 85–91% of patients) is the most common presenting feature of achalasia, as liquids require better neuromuscular co-ordination than solids for oesophageal emptying. Postures like raising the arms in an erect position increase the intraoesophagael pressure and propel food in the aperistaltic oesophagus, as the oesophagus is compressed between the spine and the manubrium sterni. Regurgitation of undigested food (occurs in 75–91% of patients) is the second most common presenting symptom. Food is regurgitated prior to reaching the stomach, unlike in gastroesophageal reflux (GERD) or gastric outlet obstruction. Retrosternal chest pain and heartburn can be seen in 40–60% of patients, which often leads to misdiagnosis as GERD and a delayed diagnosis of achalasia. Fermentation of undigested carbohydrate produces lactate and causes heartburn.13,14 Chest pain is least responsive to treatment compared to other symptoms but it can resolve spontaneously, unlike others.15 Weight loss can occur but it is not as substantial as in mechanical causes of dysphagia (e.g., oesophageal cancer or stricture). The Eckardt score is based on the degree of dysphagia, regurgitation, chest pain, and weight loss, and is used to evaluate treatment efficacy in achalasia.16 Cough and fever caused by aspiration pneumonia (8–10%) can be one of the presenting symptoms of achalasia.14 Another rare but noteworthy symptom in achalasia is impaired belching caused by compression of the membranous trachea by a dilated oesophagus and inadequate relaxation of the upper oesophageal sphincter.17 Clinical examination is usually unremarkable. Few patients may have emaciation and oral ulcerations caused by regurgitation. Diminished breath sounds, dull percussion notes, and crepitations in areas of consolidation can be found in cases of aspiration pneumonia.14
INVESTIGATIONS
Upper GI endoscopy and timed barium oesophagogram are the initial investigations to rule out mechanical obstruction. High resolution manometry (HRM) is diagnostic and helps to classify achalasia.18 The principal differential diagnoses of achalasia are GERD, pseudoachalasia, other oesophageal motility disorders, and mechanical dysphagia, which can be differentiated based on the investigations above. (Table 1A).
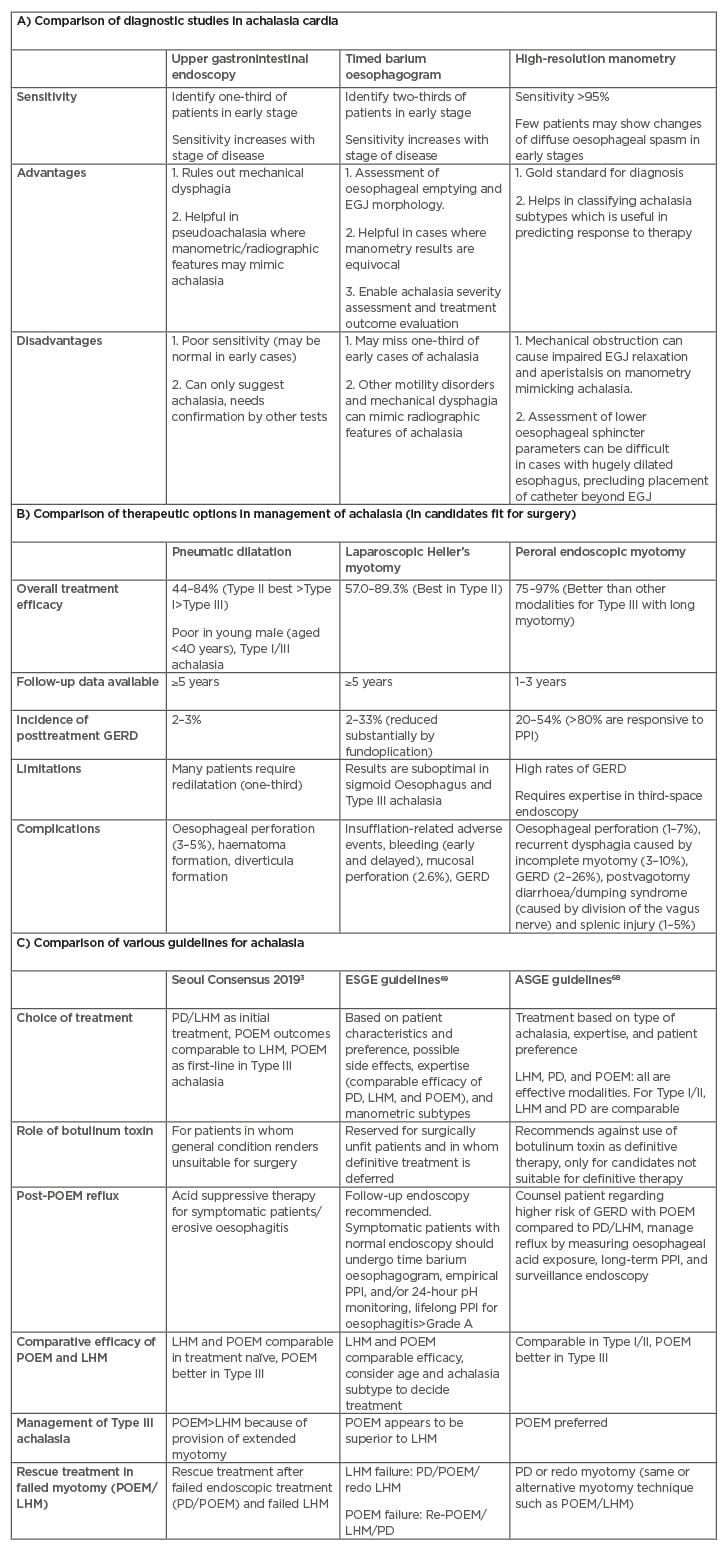
Table 1: A) Comparison of diagnostic modalities; B) treatment options; and C) various guidelines in achalasia cardia.
ASGE: American Society for Gastrointestinal Endoscopy; EGJ: oesophagogastric junction; ESGE: European Society of Gastrointestinal Endoscopy; GERD: gastroesophageal reflux; LHM: laparoscopic Heller’s myotomy; PD: pneumatic dilatation; POEM: peroral endoscopic myotomy; PPI: proton-pump inhibitors.
Upper Gastrointestinal Endoscopy
Upper GI endoscopy helps to rule out mechanical dysphagia caused by oesophageal malignancy and stricture. Endoscopy in achalasia shows a dilated and tortuous oesophagus (Figure 1A), intermittent tertiary contractions caused by spontaneous contractions of the oesophageal smooth muscles, and undigested food or liquid. The oesophageal mucosa is usually normal, but gastric stasis can cause erythema/ulceration and oesophageal candidiasis. A pulsion-type oesophageal epiphrenic pseudodiverticulum can be seen, which makes endoscopic therapy challenging. The contracted LES can be traversed with a gentle endoscope pressure unlike in malignancy/strictures.14,19
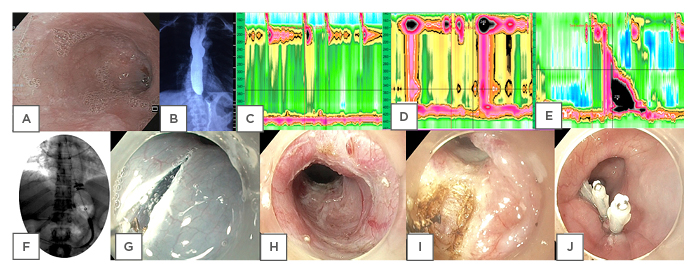
Figure 1: Diagnostic investigations and treatment modalities in achalasia.
A) Endoscopy showing a dilated, tortuous oesophagus in a case of achalasia. B) Timed barium oesophagogram at 5 minutes in a case of achalasia cardia showing retention of barium in the oesophagus. C) High-resolution manometry picture of Type I achalasia. D) High-resolution manometry picture of Type II Achalasia. E) High-resolution manometry picture of Type III Achalasia. F) Pneumatic dilatation in achalasia by Rigiflex™ balloon dilator (Microvasive, Milliford, Massachusetts, USA). G) Mucosal incision in peroral endoscopic myotomy (POEM). H) Submucosal tunneling in POEM. I) Circular myotomy in POEM. J) Closure of mucosal incision in POEM.
Timed Barium Oesophagogram
Timed barium oesophagogram (TBE) is the imaging of choice in achalasia. After swallowing 100–250 mL of barium (45% weight/volume) over 15–20 seconds, an X ray is performed at 1, 2, and 5 minutes.20 Oesophageal emptying is evaluated by the height and width of the remaining barium column in the oesophagus at 1, 2, and 5 minutes (Figure 1B). Delayed emptying of the barium from the oesophagus, tertiary contractions, and bird-beak appearance on the X-ray are the characteristic features. Post-treatment TBE is compared to pretreatment TBE to assess response to therapy. In late stages of achalasia, megaoesophagus (oesophageal diameter: >7 cm) and sigmoid oesophagus (dilated, tortuous oesophagus) can be seen, which implies decompensated disease poorly responsive to therapy.3 Oesophageal epiphrenic diverticulum can also be found, albeit rarely, on barium oesophagograms in association with achalasia. 19
High Resolution Manometry
HRM has higher sensitivity and reproducibility than conventional oesophageal manometry and hence has replaced it for diagnosis and classification of achalasia. Achalasia can be classified into three subtypes according to the Chicago classification version 3.0.2 Integrated relaxation pressure of more than an upper limit of normal with 100% failed peristalsis differentiates achalasia from other motility disorders (e.g., Jackhammer oesophagus or distal oesophageal spasm). In Type I AC, there is no oesophageal contractility or pressurisation. It represents late-stage disease with a dilated, atonic oesophagus caused by minimal oesophageal smooth muscle contractility (Figure 1C). Type II AC is characterised by panoesophagael pressurisation (in >20% swallows) between the upper and lower oesophageal sphincter, caused by disorganised oesophageal neuromuscular activity, which is indicative of intact oesophageal contractility (Figure 1D). 2,3 Thus, Type II AC represents the early stage of disease and is most responsive to pneumatic dilatation (PD).21 Type II achalasia is also the most common subtype. Type III AC is least common and least responsive to therapy, and is characterised by premature contractions (distal latency <4.5 seconds in >20% swallows) and segmental pressurisation of the distal oesophagus (Figure 1E).22
OTHER INVESTIGATIONS
As part of the work-up for endoscopic/surgical myotomy requiring general anaesthesia, complete blood count, serum creatinine, and electrolytes, liver function tests and thyroid profiles can be performed. Chest X-ray helps to identify aspiration pneumonia and CT of the chest and endoscopic ultrasound (EUS) can be useful to rule out pseudoachalasia. Marked (>10 mm), asymmetric lower oesophageal wall thickening on EUS suggest underlying malignancy.
Management
Symptomatic relief of dysphagia and associated complications are the goals of achalasia treatment. As pathophysiology is poorly understood, there is no currently available treatment directed towards pathogenetic factors. Treatment is guided by surgical risk of the patient and achalasia subtype.3 In patients with low surgical risk, pneumatic dilatation, laparoscopic Heller’s myotomy (LHM), or POEM are the mainstays of treatment. Botulinum toxin (BT)/pharmacotherapy is reserved for patients with high surgical risk/limited life expectancy.3
Botulinum Toxin
The rationale of using BT in achalasia is because of the associated blockade of acetylcholine from the presynaptic cholinergic neurons, which are relatively preserved in comparison to the selective loss of inhibitory nitrinergic ganglion cells in achalasia.23,24 During endoscopy, 100 units of BT powder is dissolved in sterile saline solution and 25 units each is injected with a sclerotherapy needle at 1 cm above the Z line/squamocolumnar junction into all four quadrants. Doses >100 units have not shown higher efficacy. In one-third of patients, LES pressure decreases and in two-thirds dysphasia is improved.25 The effect of therapy is short lived because of the growth of new cholinergic neurons. Hence, 50% of patients require reinjection after 6–12 months. Repeat injections can be technically difficult because of fibrosis from prior injections. Therefore, BT is reserved for patients with high surgical risk and limited life expectancy. BT is usually safe, although side effects such as oesophageal perforation, mediastinitis, and heartburn/chest pain has been reported.26
Pharmacological Therapy
Although several pharmacological agents like calcium channel blockers, nitrates, anticholinergics, phosphodiesterase inhibitors, and β agonists have been tested in achalasia, most of them provide short-lived benefits at most, with risk of developing tolerance on continued treatment as well as potential side effects.27 Most of the agents do not improve oesophageal peristalsis except anticholinergic cimetropium bromide, which is not widely available and seldom used.28 Most commonly used agents are calcium channel blockers (nifedipine) and nitrates (isosorbide dinitrate). Doses of isosorbide dinitrate 5–10 mg sublingually 10–15 minutes prior to every meal relaxes LES pressure by 66% for 90 minutes. Headache is a common side effect when using nitrates. Nifedipine 10 mg sublingually 10–15 minutes premeal relaxes LES by 30–40% for 60 minutes. Peripheral oedema, orthostasis, and headache are common side effects of nifedipine.29
Pneumatic Dilatation
PD is one of the recommended initial treatments for achalasia and is widely performed across various centres.3 Rigiflex™ balloon dilator (Microvasive, Milliford, Massachusetts, USA), available in three sizes: 30, 35, and 40 mm, is used for performing PD. Initially, the 30 mm balloon is used, followed by progressively larger balloons (the graded approach).30,31 After index dilatation by the graded approach, repeated dilatations on follow-up for recurrent symptoms is known as the ‘on demand approach’. After overnight fasting, the procedure is performed under fluoroscopic guidance with conscious sedation. A novel technique under endoscopic guidance without fluoroscopy has also been described.32 After passing a guidewire into the stomach by endoscopic guidance, the endoscope is withdrawn into the gastroesophageal junction (GEJ) and the length from the incisors to the GEJ is noted along the length of the endoscope. The Rigiflex balloon is passed over the guidewire corresponding to the measured distance. Radiographic contrast injection can also be done to mark the GEJ. The Rigiflex balloon is then placed across the GEJ under fluoroscopic guidance and is inflated with air to 10–15 psi until the balloon waist disappears. The waist of the balloon is seen between the two crus of the diaphragm and radiopaque marks can be seen in the balloon catheter in Figure 1F. Pressure is maintained for 1 minute. Waist obliteration, blood staining of the balloon, chest pain, and mucosal tear/widening of GEJ on postdilatation endoscopy confirms adequate dilatation.
Oesophageal perforation (3–5%), haematoma formation, and diverticula formation are the known adverse events.33 Tachycardia and/or persistent chest pain persisting for >4 hours are indicators of probable perforation and warrant a contrast eosophagogram. Conservative treatment with antibiotics and parenteral nutrition is warranted for small perforations, whereas urgent thoracotomy and repair is required if there is large perforation with free flow of contrast into the mediastinum. This is the reason why only patients with low surgical risk should be subjected to PD.33,34 Incidence of GERD post-PD is approximately 2–4%.35 Poor predictors of treatment with PD are age <40 years, chest pain, and Type III achalasia. Response rate for chest pain is approximately 50%.35 In trials comparing BT with PD, the safety and cost effectiveness of BT is offset by the requirement of repeated injections.36 According to a meta-analysis of randomised controlled trials (RCT), PD was comparable with LHM in regard to efficacy, except in young males of whom 24% required redilatation, compared to 14% with LHM.37,38 In a recent RCT, PD was shown to have a significantly lower success rate (54%) at 2 years in comparison to POEM (92%).38 The response rate of PD in the recent RCT would have increased by 76% if the 40 mm balloon was used instead of the 30–35 mm.38
Peroral Endoscopic Myotomy
POEM is a natural orifice transluminal endoscopic surgery that uses submucosal endoscopy and has revolutionised endoscopic treatment of achalasia.4 It is useful in treatment naïve, treatment failure, and Type III achalasia. General anaesthesia with endotracheal intubation and carbon dioxide insufflation is used for the procedure.39 Mucosal incision, submucosal tunnel creation, myotomy of oesophageal circular muscles, and closure of mucosal incision are the principal four steps of performing POEM (Figure 1G-1J).40 After injecting indigo carmine diluted with normal saline at approximately 13 cm proximal to GEJ, a 2 cm longitudinal incision is made anteriorly (at ‘1 o‘clock’) or posteriorly (at ‘5–6 o’clock’) with the use of a triangular tip (Figure 1G). The choice of anterior or posterior POEM depends on the operator/clinical scenario and data from RCT and meta-analysis have shown comparable results with shorter time for the posterior approach.41-46 In redo myotomy/distorted anatomy, greater curvature myotomy (at ‘8 o’clock’) can be performed. However, greater curvature myotomy is not popular as it leads to disruption of the ‘angle of HIS’, which is a predisposition to GERD. An endoscope with a transparent cap is inserted into the submucosal tunnel and extended by injection and cautery, which should be around one-third of the oesophageal circumference and extend 3 cm distally to the GEJ (Figure 1H). The mucosal layer is preserved by keeping the endoscope close to the circular muscle layer. Myotomy of circular muscles is performed by starting at 2–3 cm distal to the mucosal entry using a triangular tip knife until the longitudinal muscles are visible. This is continued between circular and longitudinal muscle fibres, up to 2–3 cm beyond GEJ (Figure 1I). Longer myotomy (>4 cm) can lead to severe erosive oesophagitis.47 Mucosal incision is closed by applying clips (Figure 1J). Current guidelines do not recommend antibiotic lavage prior to closure of the mucosal incision.48 A contrast oesophagogram is carried out at postoperative Day 1 to exclude a possible leak and to evaluate the treatment response by seeing adequacy of barium emptying. Patients tolerating an oral diet, in whom contrast oesophagogram has shown no leak, can be started on a liquid diet on postoperative Day 1, followed by a regular diet on subsequent days.40 The initial clinical success and intermediate-term efficacy after 2 years are 82–100% and 78–91% respectively.49-51
Various adverse events (0.5–3.3% of cases) with POEM include insufflation-related events (pneumoperitoneum: 6.8%; pneumomediastinum: 1.1%; and mediastinal and subcutaneous emphysema: 7.5%); bleeding, either early or delayed; and mucosal perforation in 2.6%.52 Insufflation-related adverse events can be minimised with extra low-flow carbon dioxide. A tense pneumoperitoneum (with high end-tidal carbon dioxide) can be treated with decompression by a Veress needle.40 Minor bleeding is treated with a coagrasper or electrocautery knife, whereas delayed bleeding (0.7% of cases) may require re-entry into the tunnel to coagulate the bleeding vessel.53 The risk factors for mucosal perforation are previous myotomy, submucosal fibrosis, mucosal oedema, and a long tunnel >13 cm. Closure of mucosal perforation by clips and/or Endoloops® (Johnson & Johnson, New Brunswick, New Jersey, USA), fibrin glue, OverStitch™ Endoscopic Suturing (Apollo Endosurgery, Austin, Texas, USA), or fully covered metal stents have been described.54 The prevalence of increased oesophageal acid exposure, reflux oesophagitis, and GERD symptoms after POEM ranges from 13–58%, 18–65%, and 17–40%, respectively.55 Patients should be counselled for increased risk of GERD post-POEM. However, most of the GERD after POEM are mild, asymptomatic, and proton-pump inhibitor responsive. An endoscopy should be performed at follow-up to check for reflux oesophagitis. If present, proton-pump inhibitors are the first line of management. If symptoms of reflux are present without changes of oesophagitis on endoscopy, 24 hour pH monitoring can be performed.3 Novel modifications of POEM by addition of fundoplication (POEM-F), such as in LHM, has been shown to reduce reflux in pilot studies.56 However, the need for fundoplication to treat GERD post-POEM is very low. Increased procedure time, cost, and uncertain durability are the drawbacks of POEM-F. Preservation of sling fibres, by identifying two penetrating vessels at the distal end of myotomy, have shown to reduce the degree of oesophagitis.57
Two recent RCT have demonstrated the efficacy of POEM to be superior to PD and noninferior to LHM.58,59In Type III achalasia, results of POEM are more successful than LHM because of the ability to perform long myotomy based on the length of the spastic distal segment of the oesophagus.3 POEM is preferred over LHM in patients with a sigmoid oesophagus and other spastic motility disorders.60
Laparoscopic Heller’s Myotomy
LHM is the first-line surgical therapy for achalasia; it has a response rate of 90–97% with recurrent dysphagia in 3–10% of patients. Laparoscopic incision is made anteriorly from 6 cm above the GEJ to 3 cm beyond, preserving cardio-oesophageal fat and the anterior vagus nerve. Extended gastric myotomy (3.0 cm) is associated with lower rates of repeated surgery and hence is preferred over standard gastric myotomy (1.5 cm). Post-LHM GERD with extended myotomy can be minimised by concurrent fundoplication (posterior Toupet fundoplication at 270° is a better antireflux procedure than anterior Dor fundoplication at 180°). The minimally invasive, laparoscopic approach is associated with shorter hospital stays, reduced postoperative pain, and lower disability. The laparoscopic approach is preferred over the thoracoscopic approach because of the shorter operating time and lower probability of conversion to open myotomy.
Adverse events with LHM include oesophageal perforation (1–7%) caused by inadvertent mucosal injury, recurrent dysphagia caused by incomplete myotomy (3–10%), GERD (2–26%), postvagotomy diarrhoea/dumping syndrome caused by division of the vagus nerve, and splenic injury (1–5%). In sigmoid oesophagus and Type III achalasia, the results of LHM are suboptimal. LHM has equal efficacy compared to PD with greater durability in young males.
POEM was noninferior to LHM with regard to clinical success at 2 years (83.0% and 81.7%, respectively) and associated with lower risk of serious adverse events (2.7% versus 7.3%, respectively) but higher incidence of reflux oesophagitis (44% versus 29%, respecively) according to a recent RCT.59
INTERDISCIPLINARY AND NUTRITIONAL APPROACH IN MANAGEMENT OF ACHALASIA
A multidisciplinary approach to achalasia management is crucial and should include gastroenterologists, surgeons and radiologists, dieticians, nurses, and actively participating family members.60 Highly individualised dietary management modifying food texture and fluid viscosity can help avoid malnutrition. Family members have a crucial role in encouraging adherence to dietary modifications. Malnourished patients awaiting surgery and those with poor oral intake and high risk of aspiration should be treated with tube feeding to reduce postoperative complications. In rare cases of end-stage achalasia, radiologic percutaneous gastrostomy feeding is effective. Intrajejunal feeding may be required in cases where pulmonary aspiration occurs with gastrostomy feeding.61
PROGNOSIS AND LONG-TERM COMPLICATIONS
Achalasia is a chronic neurological disorder which is not cured by LES-directed therapies and hence requires lifelong follow-up. Long-term complications include development of end-stage achalasia/megaoesophagus or oesophageal squamous cell carcinoma. Progressive dilation of the oesophagus is developed in 10–15% of patients, which leads to megaoesophagus/end-stage achalasia even posttreatment, and eventually 5% require oesophagectomy.62 The rate of squamous cell cancer is 1 in 300 patient-years, but surveillance endoscopy is not routinely recommended (number needed to detect one cancer is 400 endoscopies).63 However, after longstanding disease (10–15 years), a 3 yearly follow-up is recommended by many experts.64
Efficacy of current endoscopic/surgical modalities (POEM, PD, LHM) decreases over time. After 5 years of initial treatment, 18–21% and 25–35% patients require retreatment in LHM and PD, respectively.65,66 After POEM, at 49 months of median follow-up, 13% of patients have recurrence;67 more long-term data is required for POEM. These patients can be successfully treated with other modalities, and a small proportion will require oesophagectomy.
CONCLUSIONS
Achalasia can be diagnosed with appropriate clinical history as it is often misdiagnosed as GERD. Endoscopy and barium swallow can be helpful to rule out mechanical dysphagia. HRM is diagnostic and useful in classification, which affects prognosis and guides treatment. Treatment of achalasia should be individualised and based on surgical risk and achalasia subtype according to various guidelines (Table 1B and 1C).3,68,69 Patients with high surgical risk should undergo BT/pharmacotherapy. PD, LHM with fundoplication, and POEM are options for patients with low surgical risk. In young patients (<40 years) with Type I achalasia, POEM/LHM should be the first option of treatment as response rates to PD is low. In Type II AC, PD can be used as an initial treatment option (with LHM/POEM), as results of PD are best in Type II AC. POEM with extended myotomy is recommended for Type III achalasia (Figure 2).3,70 Upon failure of therapy, either of the three modalities can be used. Oesophagectomy should be reserved for patients with longstanding disease who are fit for surgery and have had repeated failure to various therapies.
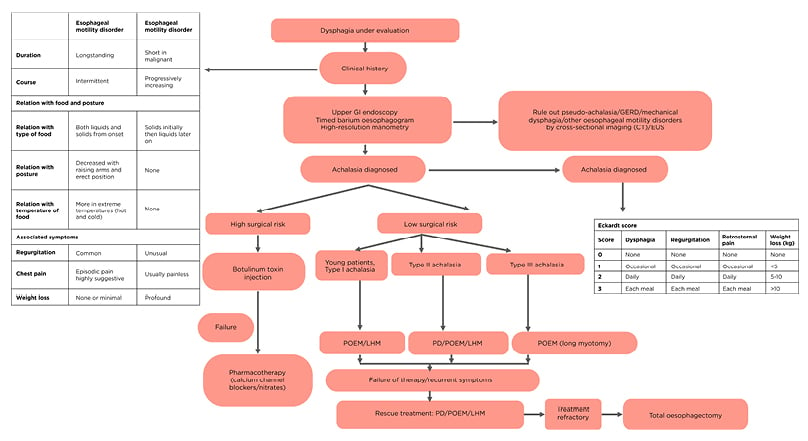
Figure 2: Stepwise diagnosis and management algorithm for achalasia cardia.
EUS: endoscopic ultrasonography; GERD: gastroesophageal reflux disease; GI: gastrointestinal; PD: pneumatic dilatation; POEM: peroral endoscopic myotomy; LHM: laparoscopic Heller’s myotomy.
Click here to view larger.