Abstract
Atrophic body gastritis is a chronic disorder characterised by atrophy of the oxyntic glands leading to reduced gastric acid and intrinsic factor secretion. Serological studies reported yearly prevalence and incidence rates between 3–9% and 0–11%, respectively. In atrophic body gastritis, the presence of parietal cells and/or intrinsic factor autoantibodies, and autoimmune diseases, such as autoimmune thyroid disease or Type 1 diabetes mellitus, are often observed. These cases are often diagnosed as autoimmune gastritis. This association has been included as part of the autoimmune polyendocrine syndrome. A frequent clinical presentation of atrophic body gastritis is pernicious anaemia, considered an autoimmune condition, arising from vitamin B12 malabsorption as a consequence of intrinsic factor deficiency. Another presentation may be an otherwise unexplained iron deficiency anaemia, as a result of iron malabsorption and consequence of reduced gastric acid secretion. To date, no universally accepted criteria are available to define autoimmune gastritis and to distinguish this clinical entity from chronic, Helicobacter pylori-driven, multifocal atrophic gastritis. In contrast with the classical perception of a silent condition, patients with atrophic body gastritis may complain of a spectrum of gastrointestinal symptoms, ranging from dyspepsia as early satiety, postprandial fullness, and epigastric pain, to gastro-oesophageal reflux symptoms such as regurgitation and heartburn. The timely diagnosis of atrophic body gastritis is important, as this condition puts patients at an increased risk of gastric cancer and other Type 1 carcinoids that may lead to micronutrient deficiencies crucial for erythropoiesis. The present review provides an update on epidemiological and clinical aspects as well as diagnosis and outcome of the disease.
EPIDEMIOLOGY AND CLINICAL PRESENTATION OF ATROPHIC BODY GASTRITIS
Atrophic body gastritis (AG) is a chronic disorder characterised by atrophy of the oxyntic glands, which leads to lack of gastric acid and intrinsic factor production, often leading to micronutrient deficiencies, such as malabsorption of vitamin B12 or iron, and consequent anaemia.1 This condition may arise from long-standing Helicobacter pylori infection or in the context of autoimmune gastritis, which harbours an increased risk for gastric neoplasias, such as intestinal-type adenocarcinoma and Type 1 gastric carcinoids, in particular when extensive intestinal metaplasia (IM) is present.
Data on the prevalence of AG derives from two methodologically different approaches, mainly performed in the general population: serological studies using surrogate markers of gastric function (pepsinogen I, or pepsinogen I/pepsinogen II ratio) or studies using gastroscopy or histology the gold standard for diagnosis of AG. Serological studies reported prevalence rates between 3% and 9%.2-7 In the population-based Kalixanda study6 performed in 2008, 6.6% of subjects had AG according to the serological biomarkers (pepsinogen I, II, and gastrin-17). Higher prevalence rates were found in Asian countries: ≤63% was reported by Zou et al.;7 this may be explained by the inclusion of not only atrophic gastritis with involvement of the body mucosa but also atrophic gastritis limited to the antrum. However, a recent Swedish serological study8 showed that AG prevalence among adults aged 35–44 years increased nearly three-fold between 1990 and 2009, but decreased by more than half in participants aged between 55 and 64 years in the same period. This unexpected trend needs to be interpreted within the limits of a serological study using a surrogate marker of AG such as pepsinogen, but the decrease of AG prevalence amongst the elderly might be explained by the stabilising seroprevalence of H. pylori. Additionally, the increase in AG stimulated by a high BMI and obesity, as an unexpected positive association between BMI and AG, was also observed. However, it remains to be established whether these novel trends of AG, considered a precursor condition of gastric cancer (GC), may ultimately affect the incidence of GC.
With regard to AG incidence, data are poor and conflicting. A systematic review published in 2010 evaluated the AG incidence in patients free of AG at the time of inclusion in the study.9 Based on 14 articles, the yearly incidence rates showed a wide range from 0.0–10.9%, probably explained by the very different clinical settings in which the AG diagnoses were made. In a meta-analysis, the ratios comparing the AG incidence in H. pylori positive patients to that in H. pylori negative patients ranged from 2.4–7.6 with a summary estimate of 5 (95% confidence interval: 3.1–8.3);9 thus, suggesting a strong relationship between incidence of AG and H. pylori infection.
In AG patients, positivity to parietal cells (PCA) and/ or intrinsic factor autoantibodies and presence of autoimmune diseases (thyroid autoimmune disease or Type 1 diabetes mellitus) are observed.10,11 Among 319 AG patients, 53% had an associated thyroid disorder; 76% of these cases were of autoimmune origin.11 Risk factors for autoimmune thyroid disease in AG patients were female sex (odds ratio [OR]: 5.6), PCA (OR: 2.5), and metaplastic atrophy (OR: 2.2). Thus, autoimmune thyroid disease and AG seem to occur in a closely linked fashion, and this link, formerly described as thyrogastric syndrome, has been included in the autoimmune polyendocrine syndrome IIIb.12 The thyroid gland and the stomach share some similar morphological and functional characteristics, likely due to their common embryologic origin.12 AG patients should therefore be screened for occult autoimmune thyroid disease, in particular women and those with positive PCA.11
A frequent clinical presentation of AG is pernicious anaemia (PA), a megaloblastic anaemia arising from vitamin B12 malabsorption as a consequence of intrinsic factor deficiency.13 Another often forgotten presentation of AG may be an otherwise unexplained iron deficiency anaemia, due to iron malabsorption as a consequence of reduced gastric acid secretion together with normal or low vitamin B12 levels.14 It has been reported that, over time, some of these patients may develop overt PA.15 The reasons for these different clinical presentations of AG patients having similar gastric histological changes are not fully understood and may have a genetic basis. A panel of single nucleotide polymorphisms related to vitamin B12 absorption investigated in AG patients with and without PA compared to healthy controls showed that a genetic variant of transcobalamin II, related to lower vitamin B12 levels, was more frequently associated in PA patients compared to controls.16 These data make plausible the idea that genetic factors contribute to determine the clinical manifestation of AG.
From a pathogenetic point of view, AG may arise as a consequence of long-standing H. pylori infection or in the context of autoimmune gastritis.1,9,13 PA, often denoted as a possible advanced stage of AG, is considered an autoimmune disorder.13 To date, no universally accepted criteria are available to define autoimmune gastritis and to definitively distinguish this clinical entity from chronic, H. pylori-driven, multifocal atrophic gastritis. Features that, theoretically, should help to differentiate between autoimmune and non-autoimmune gastritis, such as positivity to intrinsic factor and PCA, presence of enterochromaffin-like cells, PA, and absence of active H. pylori infection were observed to be present in similar proportions in patients with body-restricted atrophic gastritis (the classical histological feature of autoimmune gastritis) and those with antral and body atrophic gastritis (more commonly attributed to H. pylori infection);11,14,16-18 thus, the specific features associated with autoimmune gastritis are far from being well defined. AG is a complex condition, consisting of at least three groups: classical autoimmune H. pylori-negative AG with spared antrum, multifocal AG involving the antral mucosa with active H. pylori infection and often negative for PCA, and a third group with overlapping features (body atrophy with a normal or inflamed antrum, active or past H. pylori infection, and PCA positivity).14,16-19
The clinical suspicion of AG less frequently arises from specific gastrointestinal symptoms. Traditionally, the clinical spectrum of AG has been considered silent, even if the occurrence of symptoms in this population has been reported. A recent report showed that 56.7% of AG patients presented one or more gastrointestinal symptoms. The vast majority of patients complained of only upper gastrointestinal symptoms, and about a sixth presented lower or both upper and lower symptoms.20 Dyspepsia, subtype postprandial distress syndrome, was the most represented symptom, present in 60.2% of symptomatic patients. A smaller part of symptomatic patients complained of gastro-oesophageal reflux disease (GORD) (7.2%) and GORD with dyspepsia (17.7%). Logistic regression analysis showed that age <55 years (OR: 1.6), absence of smoking habit (OR: 2.2), and absence of anaemia (OR: 3.1) were independent factors associated to dyspepsia in AG patients. This may be relevant, as guidelines recommend gastroscopy with biopsies for dyspeptic patients >55 years of age or with alarming symptoms;21 younger dyspeptic patients without anaemia generally are not referred to gastroscopy, thus, possibly missing the diagnosis of AG. In this cohort of AG patients, the prevalence of dyspepsia was roughly two-times higher than that expected in the general population, suggesting a potential role of this condition in the arising of dyspepsia. Delayed gastric emptying has been described in patients with AG.22 A former study reported in autoimmune gastritis patients the presence of postprandial fullness, early satiety, and epigastric pain in 7.1%, 10.1%, and 35.3%, respectively; a subset of patients complained of heartburn (24.2%) and acid regurgitation (12.1%).23 Even if in AG patients the most commonly reported symptom was dyspepsia and more recently the attention has been focussed on oesophageal symptoms.
A recent paper investigated the presence of GORD with pH-impedance monitoring in a cohort of autoimmune gastritis patients;24 24% of patients were found to be GORD-positive at pH-impedance monitoring, confirming the co-presence of this condition in autoimmune gastritis. Acid gastro- oesophageal reflux rarely occurred whereas non- acid gastro-oesophageal reflux was more frequent, both being involved in the clinical presentation of some patients. Recently, a case was published presenting the unusual co-presence of two conditions, autoimmune gastritis with PA and Barrett’s oesophagus, contrasting with the commonly held belief that these two conditions are mutually exclusive.25 In this hypochlorhydric patient, who complained of dyspeptic symptoms, nausea, vomiting, and thoracic pain; the report also documented conditions typically associated with GORD, ranging from mild erosive oesophagitis to Barrett’s oesophagus, during a follow-up of 6 years, showing that gastric acid is not the sole culprit of oesophageal damage. Patients with Barrett’s oesophagus have been shown to be have significantly more oesophageal exposure to bile acids and higher oesophageal luminal concentrations of bile acids than GORD patients without Barrett’s oesophagus. Some bile salts, as deoxycholic acid, possibly deconjugated by bacteria present in the hypochlorhydric stomach, may cause DNA damage and NF-κB activation in Barrett’s cells, a combination that might predispose a patient to cancer development.26 Thus, in contrast with the classical perception of a mainly silent condition, AG patients may complain of a spectrum of gastrointestinal symptoms, ranging from dyspeptic symptoms to gastro-oesophageal reflux symptoms. Further studies are needed to identify a specific symptomatic pattern in this condition.
DIAGNOSIS OF ATROPHIC BODY GASTRITIS AND PREMALIGNANT CHANGES
AG should be clinically suspected in anaemia, dyspepsia, and in the presence of autoimmune disease, in particular autoimmune thyroid disease, but also in patients with H. pylori infection, first-degree relatives of patients with GC or Type 1 gastric carcinoids, and patients chronically using proton pump inhibitors (PPI). Figure 1 shows the clinical scenarios possibly associated with AG.
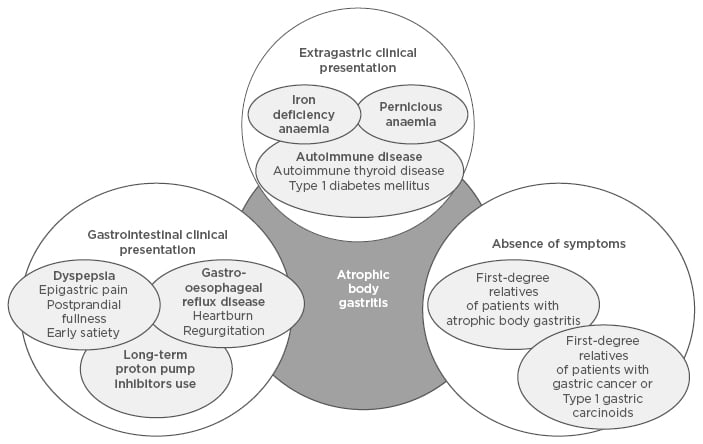
Figure 1: Possible clinical scenario of atrophic body gastritis.
The worsening of AG by long-term PPI administration was first described in H. pylori-positive Mongolian gerbils,27 and later on this negative relationship was confirmed in humans.28 The use of PPI in AG patients should be avoided for two reasons: firstly, because PPI treatment may be harmful with regard to the progression of gastric mucosal changes and increase the risk of GC as well as Type 1 gastric carcinoids; secondly, PPI-based treatment is virtually useless in this condition because gastric acid secretion is reduced due to atrophy of parietal cells; as a consequence, the target of the drug, the parietal cell H+/K+ proton pump, is lacking.
The timely diagnosis of AG is clinically important. The potential micronutrient deficiencies, for example malabsorption of iron and vitamin B12, associated with AG may lead to serious clinical consequences, such as severe chronic anaemia. This may be particularly relevant in the elderly with cardiac comorbidities, in whom a chronic slow decrease of haemoglobin may be noted, with delay leading to life-threatening complications. Vitamin B12 deficiency is a common cause of several neurological and neuropsychiatric disorders, including cognitive impairment, dementia, depression, and myelopathy with or without an associated neuropathy. Vitamin B12 deficiency has been associated with neurologic, cognitive, psychotic, and mood symptoms, and it should be accurately diagnosed and treated early to prevent irreversible structural brain damage and reduce morbidity among elderly patients.29,30 Iron deficiency has also been recognised as having a negative impact on cognition, behaviour, and motor skills.31 Considering that in AG both iron and vitamin B12 deficiencies may coexist, a timely and correct diagnosis of this condition is particularly important in the elderly.
AG increases the risk for GC and Type 1 gastric carcinoids. Although the GC incidence has declined over the past decades, especially in Western countries, the mortality rate due to GC remains high.32 Detection and surveillance of patients with premalignant conditions, including AG and IM, could lead to detection of lesions at an early stage.33 In Western countries, the gold standard for AG diagnosis is the histopathological evaluation of gastric biopsies, which should include at least five biopsy specimens from antral and body mucosa.34 This method may be impractical for routine practice because of the time, efforts, and costs required to obtain biopsies and pathology results. The recent position statement from the British Society of Gastroenterology (BSG) on quality standards in upper gastrointestinal endoscopy confirms the need to obtain two non-targeted biopsies from the antrum and body, and one from the incisura, as separate samples. This is carried out in addition to targeted biopsies of any visible lesions, where endoscopic features suggest potential gastric atrophy or metaplasia, in order to confirm this diagnosis and to exclude dysplasia, albeit the grade of evidence is weak.35 In Japan, gastric mucosal atrophy is commonly diagnosed by endoscopic appearance, with endoscopic findings of atrophic mucosa consistent with histological findings of atrophic gastritis.36
Magnifying narrow-band imaging (M-NBI) has been reported to be useful to predict the presence and distribution of IM in the gastric body. Narrow band imaging is an electronic chromoendoscopy permitting an enhanced visualisation of microvascular architecture and microsurface structure. Several studies reported a good correlation between narrow band imaging appearances and pathology in IM and GC.37 In M-NBI of the stomach, a light-blue crest is widely known to be a useful endoscopic marker of IM, and, more recently another marker, a white opaque substance without a light-blue crest, has been observed.38 This innovative endoscopic technique presents a high diagnostic value for gastric precancerous lesions with a high specificity, thus possibly permitting targeting biopsies to optimise diagnostic yield with respect to random biopsies protocols.39 The above-cited BSG position statement recommends a careful examination of the stomach with white light endoscopy to be performed as a minimum, with evaluation with chromoendoscopy considered.35
With regard to the pathological diagnosis of AG, several classifications have been proposed for AG and preneoplastic changes. The updated Sydney System is more frequently used, which combines topographic, morphological, and aetiological information to standardise histological reporting.34 More recently, operative links for gastritis and IM assessment have been proposed for the staging of gastritis and IM.40 Unfortunately, classifications are often difficult to use in clinical practice. An Italian survey showed that in routine practice only one third of histology reports were created adhering to the Sydney System, showing that guidelines are poorly observed in clinical practice, possibly representing a critical element for GC surveillance strategies.41 The full adherence to the Sydney System significantly increased the probability of detecting gastric IM (OR: 9.6) and atrophy (OR: 1.9),41 thus underlining its potential benefits.
A non-endoscopical diagnostic approach for AG diagnosis is represented by the serological gastric biopsy, including serum pepsinogen I and II and gastrin as well as H. pylori antibodies. The diagnostic potential of the serum markers in predicting the mtopography and severity of gastric mucosal disorders has been established.42,43 According to a meta-analysis, a panel of serological markers (gastrin 17, pepsinogen I and II, and H. pylori antibodies) showed a 70.2% pooled sensitivity and a 93.9% pooled specificity in non-invasive diagnosis of AG.44
PCA may be considered serological markers of AG, whose potential role in the non-invasive screening or diagnosis is underestimated. PCA are immunoglobulin G against the parietal cell H+/K+ ATPase, are mainly considered serological markers of autoimmune gastritis, and are used to screen patients with other autoimmune disorders for this condition.14,19 PCA, in particular against the ATP4A and ATP4B subunits of the gastric proton pump H+/K+ ATPase, have recently been shown to be virtually always present in patients with a known diagnosis of AG, by using an innovative luminescent immunoprecipitation system (100% sensitivity for the ATP4A and 95% sensitivity for the ATP4B subunits), and thus represent reliable markers of oxyntic mucosa atrophy.45 The assessment of immunoglobulin G autoantibodies against ATP4A and/or ATP4B subunits may be proposed as a biomarker not only for autoimmune gastritis, but also for other forms of AG, and positive patients should be advised to undergo gastroscopy with biopsies in order to establish AG diagnosis and to rule out neoplastic complications of this condition.
OUTCOME OF ATROPHIC BODY GASTRITIS: A PRECANCEROUS CONDITION
Gastric mucosal atrophy and IM are known to confer a high risk of GC, thus representing precancerous conditions. The development of the intestinal-type gastric adenocarcinoma represents the end step of an inflammation–metaplasia–dysplasia–carcinoma sequence, called Correa’s cascade.46,47 The intragastric distribution of premalignant changes of the gastric mucosa is one determinant of the GC risk: cases of oxyntic gland atrophy and/or IM distributed in a multifocal pattern, including the lesser curvature of the corpus and fundus, are called multifocal atrophic gastritis, and this phenotype, described as extensive, has been associated with a higher risk of GC. The concept of gastritis of the carcinoma phenotype proposes that the corpus-predominant gastritis increases the risk of GC, likely due to changes in the intragastric milieu (increased pH, reduced ascorbic acid, and scavenging of nitrites).48,49
Gastric dysplasia constitutes the penultimate stage of the gastric carcinogenesis sequence and is to be considered a direct neoplastic precancerous lesion. The Padova and Vienna classifications are tools to standardise the terminology for the morphological spectrum of gastric dysplastic lesions. The World Health Organization (WHO) classification50 provides the diagnostic categories: 1) negative, 2) indefinite, 3) low grade, 4) high grade intraepithelial neoplasia/dysplasia, and 5) intramucosal invasive neoplasia or intramucosal carcinoma.
AG is also associated with Type 1 gastric carcinoids, which are gastrin-dependent, well-differentiated tumours with a generally benign behaviour, representing up to 80% of all gastric carcinoids.51 Hypergastrinaemia due to AG is the main pathogenetic factor for Type 1 gastric carcinoids acting as a growth factor for enterochromaffin-like cells; through a multistep process passing from hyperplasia to dysplasia, carcinoids may develop.51
Many efforts have been made to quantify the risk of gastric neoplasms in AG patients. A varying progression rate of AG to GC, up to 2% per year, has been reported at follow-up periods of up to 16 years.52,53 A systematic review showed in AG patients with PA an estimated seven-fold relative risk of GC.54 Data on long-term incidence of Type 1 gastric carcinoids are scarce; a cohort study reported an annual incidence rate for Type 1 gastric carcinoid of 0.4%.53
In AG patients, the cost-effectiveness of regular endoscopic follow-up for GC surveillance is not established. The Management of precancerous conditions and lesions in the stomach (MAPS) guidelines recommend a surveillance for GC for patients with extensive atrophic gastritis or IM,55 but these guidelines are not addressed to PA patients because PA is not considered to be part of the precancerous cascade.46 According to the above- reported studies,53,54 a different clinical management of AG patients with or without PA does not seem justified. A cost analysis by surveillance endoscopy in AG in Italy showed that at the 361 surveillance gastroscopies, 20 neoplasias were detected, corresponding to a number-needed-to-screen of 19 and a cost-per-gastric-neoplastic-lesion of €2,945. By restricting surveillance to PA patients, the number-needed-to-screen and the cost-per-neoplasia was reduced to 13.8 and €2,139, respectively, and still detected 74.0% of neoplasias, thus confirming the association of PA with GC and supporting the need of surveillance in this condition. PA may be viewed as one of the risk factors allowing an efficient allocation of endoscopic surveillancein AG in a low-risk country.56
With regard to the combined risk of GC and carcinoids, a recent study57 assessed the occurrence of GC and carcinoids in a cohort of AG patients at long-term follow-up from 4 years onwards. The annual incidence rates per person-year were 0.25%, 0.43%, and 0.68% for GC, dysplasia, and Type 1 gastric carcinoids, respectively; the incidence rates of GC and Type 1 gastric carcinoid were the same (p=0.07), indicating that AG patients are similarly exposed to both risks.
The occurrence of GC in patients with Type 1 gastric carcinoids was described in 23.0% (4 of 17) patients with Type 1 gastric carcinoids in a median follow-up period of 6 years.57 Three cases were intestinal-type adenocarcinomas and one was signet ring cells diffuse GC, localised in three cases in the antrum. Thus, the surveillance of Type 1 gastric carcinoids patients with an accurate bioptic sampling of antral mucosa seems of benefit. Long-standing hypergastrinaemia may explain why patients with Type 1 gastric carcinoids might develop more frequently GC. Hypergastrinaemia has been proposed in many models of gastric carcinogenesis and seems to be a common causative factor in otherwise different circumstances.58 Also, the long- term conservative management of Type 1 gastric carcinoids exposes these patients to a higher risk of GC.
CONCLUSION
AG is an underdiagnosed and mainly benign condition, which may clinically present with upper gastrointestinal symptoms, but also with extragastrointestinal signs or symptoms. This condition may harbour two underhand consequences; firstly, the increased risk for two types of gastric neoplasms, GC and Type 1 gastric carcinoids; secondly, the occurrence of erythropoietic micronutrient deficiencies potentially leading to anaemia due to iron or vitamin B12 deficiency which need prompt treatment. Patients with clinical suspicion of AG should undergo serological screening by serum pepsinogen I and II, gastrin, and/or H. pylori antibodies, in order to better address gastroscopy. Another useful screening tool of AG is represented by PCA assessment. The definite diagnosis still delays on gastroscopy and pathological evaluation of multiple body and antral biopsies, possibly staged by the updated Sydney System, operative link for gastritis, and IM assessment. Innovative endoscopy techniques, such as M-NBI, may in a near future allow to target biopsies at surveillance endoscopy improving the early detection of gastric neoplasms.








