INTRODUCTION
Immune checkpoint inhibitors (ICI) are a novel class of anticancer therapy that have been transformative in treating a diverse range of cancers, extending survival in some patients, and producing significant and durable tumour responses. ICI enhance immunological responses against tumour cells by inhibiting receptor-ligand interactions in immune checkpoint pathways, which may be subverted by tumour cells to prevent their destruction by cytotoxic T cells. The two classes of ICI currently in routine clinical use are monoclonal antibodies targeting CTLA-4 and PD-L1.1,2
Use of ICI is associated with a novel range of side effects, termed immune-related adverse events (IRAE). These can affect any organ system, and are thought to arise from immune-mediated attack on healthy host tissues following reduction in self-tolerance due to immune checkpoint blockade. Immune-related hepatitis (IRH), usually manifesting as elevation of hepatocellular enzymes, may occur in 2–25% of patients, depending on ICI class or combination.1,2
Management of IRAE represents a particular clinical challenge. Management guidelines, such as those from the European Society for Medical Oncology (ESMO),1 were initially developed based on clinical trial protocols and case reports/series, and have been revised to reflect expertise gained from increased experience in managing IRAE. While these management algorithms appear generally effective for most patients, they have never been evaluated prospectively, and many areas of uncertainty remain.
This article will review some of the challenges in management of patients with IRH, focusing on areas where hepatologists are often asked to assist with management, but where little evidence exists to guide decision-making (Figure 1). Specifically, this includes diagnosis and role of liver biopsy; management of corticosteroids (CS) and other immunosuppressive therapies (IST); risks of ICI rechallenge in patients with IRH; and ICI treatment in special populations, such as those with viral hepatitis.
Figure 1: Summary of key challenges in management of patients with immune checkpoint inhibitor- associated hepatitis.
ICI: immune checkpoint inhibitor; IST: immunosuppressive therapy.
DIAGNOSTIC CHALLENGES
Several biomarkers associated with increased risk for IRAE have been identified, including cellular markers, such as neutrophil to lymphocyte ratio; cytokine/chemokine expression, including IL-6, IL-17, CXCL 9, CXCL 10, and CXCL 11; single nucleotide polymorphisms; and gut microbiota. However, no single biomarker reliably predicts IRAE development.3 The association between IRAE and anticancer efficacy limits the identification of a diagnostic biomarker, such that a toxicity biomarker may also indicate cancer response.4 Therefore, IRH currently remains a diagnosis of exclusion. Initial investigation and management are usually conducted by the patient’s primary oncologist. However, early involvement of a hepatologist is desirable, both to facilitate consideration of alternate causes for liver injury before initiation of IST, which complicates the clinical picture, and to build expertise in managing these complex patients.
The role of biopsy in diagnosis has been a topic of debate. Biopsy is often deferred, due to concerns that it will delay initiation of therapy. Histopathological findings can vary widely, with no pathognomonic features of IRH described. Conversely, a biopsy may be helpful to exclude an alternate/concurrent disease process. A consensus view is that biopsy can usually be reserved for atypical presentations, or patients who fail to respond to treatment with IST (Figure 2).5
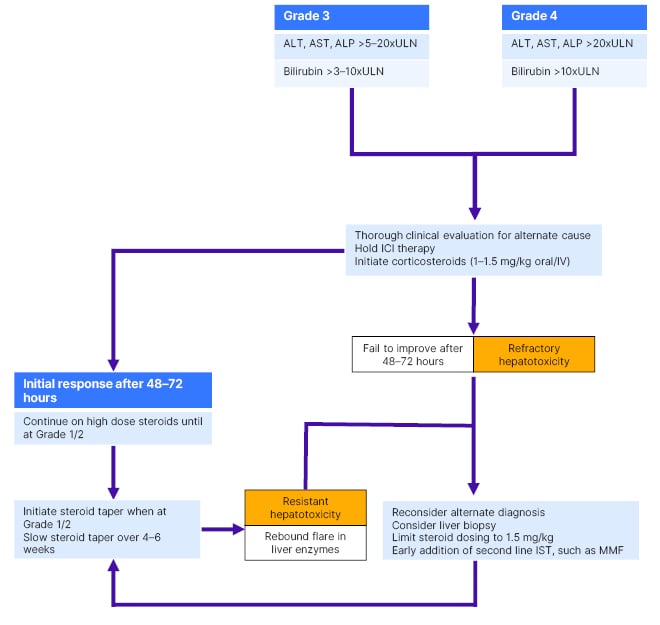
Figure 2: Suggested management algorithm for patients with high-grade immune checkpoint inhibitor- associated hepatitis.
ICI: immune checkpoint inhibitor; IST: immunosuppressive therapy.
HOW SEVERE IS ‘SEVERE’?
Reflecting the development of management guidelines from clinical trial protocols, severity of IRH is graded using Common Terminology Criteria for Adverse Events (CTCAE; Table 1). Under these criteria, severity may be adjudicated biochemically rather than clinically, based on the extent of liver enzyme elevation regardless of liver function. This contrasts with other severity assessment tools, such as the Drug Induced Liver Injury Network (DILIN) severity index, which incorporates markers of liver function into severity grading (Table 2).6 For example, an asymptomatic patient with ALT elevation 5x upper limit of normal, but normal bilirubin and international normalised ratio (INR; a relatively common presentation of IRH) would be adjudicated as high grade (Grade 3) by CTCAE criteria, but only mild by DILIN criteria. Using CTCAE to manage IRAE is embedded within guidelines and clinical practice, but may overestimate liver injury and lead to overtreatment. Whether adopting alternate DILIN severity assessments to stratify liver injury and guide therapy would improve outcomes in patients with IRH remains unknown.
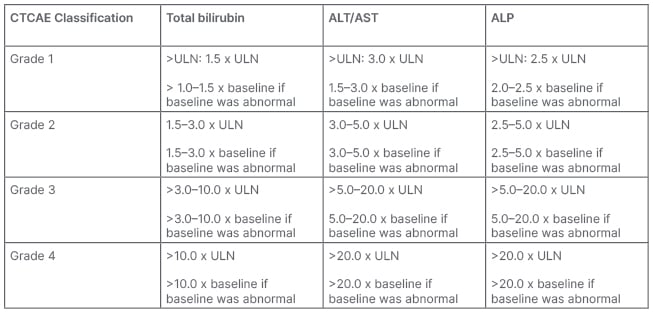
Table 1: Common Terminology Criteria for Adverse Events (CTCAE) for bilirubin and liver enzyme elevations.
Version 5.0, November 2017.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP, alkaline phosphatase; CTCAE: Common Terminology Criteria for Adverse Events; ULN: upper limit of normal.
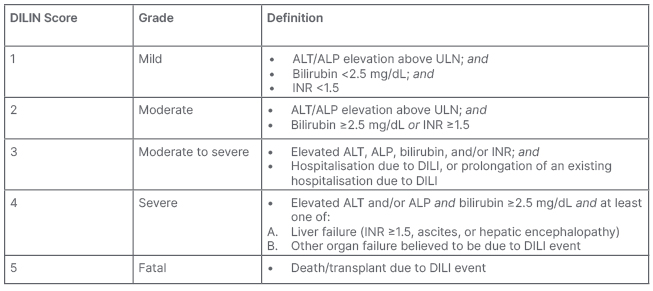
Table 2: Drug Induced Liver Injury Network (DILIN) severity index for adjudicating the severity of drug induced liver injury.6
ALT: alanine aminotransferase; ALP: alkaline phosphatase; DILI: drug-induced liver injury; DILIN: Drug Induced Liver Injury Network; INR: international normalised ratio; ULN: upper limit of normal.
DOES EVERYONE NEED STEROIDS?
The cornerstone of management for high-grade IRH is stopping ICI and treatment with high-dose CS. Figure 2 describes a proposed management algorithm. Little natural history is reported on evolution of IRH in the absence of CS. However, even high-grade IRH may resolve in some patients after simply holding ICI.7 Management strategies have been proposed where CS are reserved for patients with synthetic dysfunction and/or severe histological inflammation,7 but these have proven challenging to implement, perhaps due to concerns over delaying CS treatment in patients who do not improve. ESMO guidelines emphasise prompt diagnosis of IRAE, based on a broad consensus that early treatment is important to improve outcomes.1 However, the default position of high-dose CS for all patients with high-grade IRH probably overtreats some patients, and will make it harder to conduct research into biomarkers associated with spontaneous resolution.
STEROID DOSING: BENEFITS VERSUS HARMS
ESMO guidelines recognise the risk for steroid-associated adverse events in patients treated with very high doses of CS, and emphasise CS treatment at the lowest dose and for the shortest period.1 Specific management guidance for IRH recommends a “step up” approach, stratified according to grade of liver injury, starting with lower doses (0.5–1.0 mg/kg) in Grade 2 IRH, or higher doses (1-2 mg/kg) in Grade 3 or 4 IRH, and increasing the dose if necessary. In practice, there is considerable variation in approach to CS dosing amongst individual physicians, with some preferring a “top-down” strategy, starting with high CS doses (up to 2 mg/kg at initiation), whilst others prefer to “step up”, starting with lower doses (0.5–1.0 mg/kg), and increase the dose if response is poor.
A recent large retrospective study suggested that very high doses of CS (>1.5 mg/kg) do not confer any additional advantage in terms of time to resolution of liver injury, or need for additional second-line IST in patients with high-grade IRH, but were associated with increased rates of steroid-associated side effects.8 Therefore, the management strategy described in Figure 2 avoids escalating steroids to doses >1.5 mg/kg, and proposes earlier introduction of second-line IST in patients who do not respond to lower doses. This approach should be prospectively evaluated for effect on resolution of hepatitis and reduction of treatment-associated harms.
WHEN THE STEROIDS DON’T WORK, WHAT NEXT?
Most patients with IRH resolve their liver injury with CS treatment. However, retrospective series suggest that 10–30% of patients develop resistant hepatitis, where liver enzymes initially respond to CS but then flare with CS taper; or refractory hepatitis, where there is no response to CS treatment.8,9 These patients often require additional IST, and prompt recognition of CS resistance/refractoriness with introduction of second-line treatment may ultimately reduce exposure to high-dose CS and associated side effects. ESMO guidelines recommend that a second agent is initiated after 48–72 hours in patients with high-grade IRAE, not responding to CS alone (Figure 2).1
Mycophenolate mofetil (MMF) is the most frequently used second-line agent for IRH, likely due to its ease of use, favourable toxicity profile, and demonstrated efficacy in case reports/series. However, MMF-refractory cases are reported. Tacrolimus has been successfully used as third-line therapy in these cases. However, whether an alternate agent would provide a more consistent response as second-line therapy is unknown.5 Plasma exchange and anti-thymocyte globulin have been used with some success in patients with liver failure.1 Azathioprine is suggested in some practice guidelines,1,2 presumably extrapolated from management of idiopathic autoimmune hepatitis. However, there is very little supporting data, and its delayed onset of action seems counterintuitive in acute IRH. Anti-TNF blockade with infliximab has shown efficacy in cases of IRH. Still, societal recommendations that infliximab is contraindicated in IRH will likely limit more widespread evaluation.1,2 This is based on the rare association between infliximab and immune-mediated hepatitis, usually associated with more prolonged infliximab exposure than in IRAE management.
Although management of IRAE is a priority in patients who are ICI-treated, concerns that broad spectrum IST may compromise the cancer response to ICI have led to proposals for individualised treatment regimens, targeting key inflammatory components implicated in specific IRAE.10 Anti-cytokine therapies, such as tocilizumab, may potentially treat IRAE without compromising anti-tumour immunity. There are increasing case reports of tocilizumab in treatment of IRH, with many, although not all, reporting favourable outcomes.1,11 Rational development of a selective IST approach for IRH is desirable, ideally guided by further translational research providing greater insights into its pathophysiology. Meanwhile, earlier use of tocilizumab may be an attractive strategy to treat IRH without compromising cancer response, reserving more broadly immune-suppressing treatments as second- or third-line, and warrants further evaluation. A Phase II study of ipilimumab, nivolumab, and tocilizumab for advanced melanoma is currently underway, to investigate whether addition of tocilizumab is associated with a decrease in IRAE and/or increased anti-cancer efficacy.12 As up to 25% of patients treated with ipilimumab/nivolumab may develop IRH,1 the study’s results may help further define the role of tocilizumab in its prevention and treatment.
EXIT STRATEGIES: HOW TO MANAGE THE TAPER
Most cases of IRH resolve with IST without evolving into chronic hepatitis. There are significant variations in practice regarding initiation and rate of CS dose taper. It is not clear if resistant hepatitis may be associated with early initiation or rapid rate of taper. There are clear benefits to avoiding steroid resistance, as this prolongs exposure to IST, increases potential for CS-associated side effects, and may delay resumption of anticancer therapy.
ESMO guidelines recommend that in Grade 2 IRH, CS should be tapered over 2 weeks after liver injury has improved to Grade 1. For Grade 3 or 4 IRH, tapering over 4–6 weeks is recommended once liver injury has improved to Grade 2.1 Although helpful in their specificity, these tapering regimens are empirical, and would benefit from prospective evaluation to establish best practices in CS management.
CAN IMMUNE CHECKPOINT INHIBITORS BE RESTARTED?
Societal guidelines have recommended permanent discontinuation of ICI following symptomatic Grade 3 or any Grade 4 IRH, due to risk of recurrent severe toxicity.2 However, other treatment options may be limited, and especially as IRAE may be associated with improved cancer response to ICI therapy, patients may benefit from re-treatment.
ESMO guidelines suggest consideration of three possible scenarios for ICI resumption: class switch from anti-CTLA-4 to anti-PDL1, or vice versa; resumption of the same class agent or same molecule; or resumption of ICI with prophylactic immunosuppressive therapy. Data to support any of these options is limited, and individualised discussion of rechallenge by a multidisciplinary team is recommended.1
A recent prospective study confirmed the overall safety of rechallenge in patients with high-grade IRH, reporting a recurrence rate of 35%. Most patients in this study developed IRH after treatment with anti-PDL1 monotherapy, and most (78%) were re-treated with the same drug.13 In general, severity of the recurrent episode of IRH was similar to the index episode, although one patient developed severe toxicity with liver failure. At the time of ICI rechallenge, 34% were still receiving prednisone (5–10 mg/day) for the previous IRAE, but this did not appear to reduce the risk for recurrence of IRH.13 Based on limited data, ICI rechallenge appears generally safe in patients with high-grade IRH. There is currently no proven benefit to prophylaxis with CS or other IST.
VIRAL HEPATITIS
All patients should be screened for hepatitis B (HBV) and hepatitis C (HCV) infection before starting ICI.14,15 Most ICI registration trials excluded patients with chronic viral hepatitis, except trials of ICI for hepatocellular carcinoma. Data from these studies, and subsequent real-world data, confirm the overall safety of ICI in patients with chronic viral hepatitis without an adverse impact on cancer outcomes, or risk for IRAE.1,15
In theory, blocking the PD-1/PD-L1 axis in patients with chronic viral hepatitis may partially restore the function of virus-specific CD8 T cells, leading to enhanced viral recognition, and potentially bystander liver injury due to the enhanced antiviral immune response. Damage to hepatocytes may also cause release of virus into the circulation, resulting in viral reactivation.15 In patients with chronic HBV infection, reactivation risk may be as high as 21%, but is substantially reduced by treatment with prophylactic antivirals. Therefore, all patients with chronic HBV should receive antiviral prophylaxis during ICI treatment, and 6–12 months after.14,15 There is little data on risk of reactivation in patients with resolved HBV infection, and current recommendations are for careful monitoring without routine use of antiviral prophylaxis.14 It is important to recognise that if patients require CS to treat any IRAE, this may increase risk for HBV reactivation, and the need for antiviral prophylaxis should be re-evaluated.14
Some studies have observed a fall in HCV viral load in patients with chronic HCV treated with ICI. HCV reactivation has rarely been described, manifesting as an elevation in liver enzymes.15 There are now several reports of successful HCV treatment with direct-acting antivirals (DAA) concomitant with ICI therapy, so chronic HCV infection is not a contraindication to proceeding with ICI therapy.1,15 Whether there is a benefit to initiating DAA therapy as prophylaxis and optimal DAA treatment in the event of HCV reactivation would benefit from further investigation.
SUMMARY AND CONCLUSIONS
As ICI use expands, so will the incidence of IRAE, including IRH. Diagnostic biomarkers are currently lacking, which specifically identify IRH and stratify severity. Current biorepository studies may help to identify biomarkers that fulfil this need. Increased experience in IRH management has led to refinements in successive iterations of clinical practice guidelines, although many recommendations remain empirical, and lack rigorous evaluation. Proposed management (for example, as described in Figure 2) is moving towards a strategy of therapeutic parsimony, with reduced CS dosing, and earlier introduction of IST to minimise the risk for steroid-associated side effects. At the same time, a new therapeutic paradigm is emerging in IRAE management to decouple IRAE from cancer response, through the use of more selective IST. MMF is widely used as second-line IST for IRH, with a generally good effect. There are increasing reports of successful use of tocilizumab as third-line therapy, and results from ongoing trials may indicate whether it should be used earlier in the treatment pathway. Finally, greater understanding of the pathophysiology underlying IRH may inform selective IST treatment strategies to optimise outcomes for patients treated with ICI therapies.







