![]()
Corrigendum: Conservative Management in a Patient with Recurrent Boerhaave Syndrome
Authors: Alexander J Kaye, Daniel Rim, Sushil Ahlawat
Original Citation: EMJ Gastroenterol. 2022; DOI/10.33590/emjgastroenterol/22-00018. https://doi.org/10.33590/emjgastroenterol/22-00018.
Date Correction Published:
In the article by Alexander J Kaye et al. in EMJ Gastroenterology 11.1, pages 73–79, the following reference was cited in error: 25. Vickers NJ. Animal communication: when I’m calling you, will you answer too? Curr Biol. 2017;27(14):R713-5. This reference has now been removed and the manuscript has been renumbered accordingly.
The authors apologise for the error and any inconvenience caused.
![]()
Authors: *Alexander J Kaye,1 Daniel Rim,1 Sushil Ahlawat2
1. Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA
2. Division of Gastroenterology and Hepatology, Rutgers New Jersey Medical School, Newark, New Jersey, USA
*Correspondence to [email protected]
Disclosure: The authors have declared no conflicts of interest
Author Contributions: Kaye wrote the article; Rim and Ahlawat edited the article; and Ahlawat is the article guarantor.
Received: 17.01.22
Accepted: 16.03.22
Keywords: Boerhaave syndrome, conservative management, oesophageal perforation, oesophageal tear.
Citation: EMJ Gastroenterol. 2022; DOI/10.33590/emjgastroenterol/22-00018. https://doi.org/10.33590/emjgastroenterol/22-00018
Abstract
Boerhaave syndrome (BS) is a full thickness oesophageal tear. Most cases require surgical correction. In rare cases, conservative management can be attempted. This case describes a 30-year-old patient with a history of BS 9 months prior, managed conservatively at that time, presenting with 2 days of vomiting. Their vitals and physical examination were within normal limits. Imaging revealed extensive air tracking along the mediastinum, oesophagus, and bilateral neck and chest wall consistent with BS. Gastrographin and barium oesophagrams showed no contrast extravasation. The patient was successfully treated with conservative measures including pantoprazole, piperacillin-tazobactam, cessation of oral intake, and parenteral nutrition. This case represents one of only two documented cases of recurrent BS in an adult patient in which both cases were managed conservatively. Of these two cases, this is the first report with the second episode of BS occurring more than 4 weeks after the first episode. Additionally, unlike the other recurrent BS case that was conservatively managed, this patient lacked any known comorbid risk factors for development of BS. While the management of this case resembles the care provided in some prior case reports, the significant variation and lack of standardisation in the approach of conservative BS treatment posed a significant challenge in developing a therapeutic plan. Given the absence of high-quality studies about conservatively managed BS, an increased sample size of case reports detailing their non-procedural management approaches and their subsequent outcomes would hopefully eventually clarify an optimal treatment regimen, and allow for standardisation of conservative BS treatment.
Key Points
1. Boerhaave syndrome is a full thickness oesophageal tear, associated with high mortality if not identified and treated promptly. In most cases, Boerhaave syndrome is managed either endoscopically or surgically to repair the tear.2. Rarely, the condition is managed conservatively. This case presents one of two documented in the literature in which recurrent Boerhaave syndrome in an adult patient was managed conservatively on both occasions. Uniquely, in this case, the Boerhaave syndrome recurrence occurred more than 4 weeks after the index event.
3. There is a lack of standardised guidance for the non-procedural management of Boerhaave syndrome. Further studies with high quality data are required to provide an evidence-base for the informal criteria currently used to help guide clinician decision making and determine the optimal conservative management strategy.
INTRODUCTION
First described by Herman Boerhaave in 1724, Boerhaave syndrome (BS) is rare and involves a full thickness tear of the oesophagus.1 The annual incidence of BS is approximately 3.1 cases per 1 million individuals.2 Risk factors for BS include alcohol abuse, excessive indulgence of food, gastroesophageal reflux, peptic ulcer disease, vomiting, neurologic disease, and hiatal hernia.3,4 Early identification and treatment of BS is crucial, as delay in treatment is associated with high mortality.5 Nearly all cases require correction of the tear, either surgically or endoscopically. In rare cases, conservative management can be attempted.6 Here, the authors report a patient with a history only notable for BS 9 months prior, who presented with another episode of BS.
CASE REPORT
A 30-year-old patient with a past medical history of BS, and an abdominal stab wound 11 years prior, presented with approximately 48 hours of diffuse abdominal pain worst in the upper abdomen, nausea, vomiting, and decreased oral intake. The past medical history of BS occurred 9 months prior, at which time the patient presented for vomiting. CT imaging was consistent with a lower third oesophageal injury. The patient was managed conservatively with 3 days of piperacillin-tazobactam and kept 1 day nil per os (NPO) with advancement of the diet over 3 days. The history was also notable for the patient being an occasional user of alcohol, although they had drunk 2–3 alcoholic beverages immediately prior to the onset of their symptoms. Notably, they denied fevers, chills, lightheadedness, dizziness, or chest pain. Their presenting vitals were a temperature of 98.6 °F, blood pressure of 124/61 mmHg, pulse of 85 beats per minute, respiratory rate of 18 breaths per minute, and oxygen saturation that was 100% on room air. The physical examination was notable for a well-appearing, alert, and oriented adult in no acute distress, and a well-healed abdominal surgical scar. No chest wall crepitus, Hamman’s sign, or abdominal tenderness were appreciated, and more generally no focal findings on exam were found. Laboratory results were notable for a white blood cell count of 16.1 x 10*3/µL (normal range: 4×10*3–11×10*3 /µL) with a differential of 87.1% neutrophils, 5.0% lymphocytes, 7.8% monocytes, 0.0% eosinophils, and 0.1% basophils, serum creatinine of 2.0 mg/dL (baseline: 0.8–1.0 mg/dL), blood urea nitrogen 37 mg/dL (normal range: 6–20 mg/dL), and serum magnesium of 1.5 mg/dL (normal range: 1.6–2.5 mg/dL). Serum sodium was slightly elevated from baseline, and both lactic acid and procalcitonin were within normal limits. CT scans of the chest, abdomen, and pelvis (Figure 1) demonstrated extensive gas tracking along the bilateral neck, chest wall, and all compartments of the mediastinum, but most prominently in the region of the lower third of the oesophagus above the level of the diaphragm. No overt oesophageal wall abnormality was detected. The CT imaging further demonstrated that the central tracheobronchial tree was patent and there was no direct evidence of alveolar rupture or gas traveling along the bronchovesicular interstitial sheaths. Cardiothoracic Surgery and Gastroenterology services were consulted by the emergency department and neither recommended interventions. Specifically, the Gastroenterology service deferred upper endoscopy due to concern that the required air insufflation could worsen a perforation. Gastrografin oesophagogram and subsequent barium oesophagogram (Figure 2) showed subcutaneous emphysema of the bilateral neck and superior chest wall, but no evidence of oesophageal contrast extravasation, intramural tracking, or penetration. The patient was administered 2 L of lactated ringers, 4 mg of ondansetron, 2 g of magnesium sulfate, and started on piperacillin-tazobactam. Within 2–3 hours following these interventions, the patient reported complete resolution of symptoms. The patient was admitted to the Internal Medicine service for further care. The patient’s laboratory abnormalities resolved with the following set of laboratory studies the subsequent day.
Figure 1: CT scans of the chest, abdomen, and pelvis demonstrated gas tracking from the neck (A) through the areas surrounding the first (B), second (C), and third (D) parts of the oesophagus, with more pronounced air tracking around the third part.
No oesophageal wall abnormality was appreciated. The central tracheobronchial tree was patent and there was no evidence of alveolar rupture or gas moving along the bronchovesicular interstitial sheaths. The orange arrows indicate the gas tracking and the green arrows indicate the oesophagus.
The patient met the Cameron criteria for conservative management of BS, which was discussed with the patient who agreed to non-procedural management.7 The patient was kept NPO for 7 days. Nasogastric and nasojejunal tubes were considered, and thought to be too risky in the setting of the recent oesophageal perforation. Interventional Radiology was consulted for placement of a peripherally-inserted central catheter, after which total parenteral nutrition was initiated. Additionally, the patient received pantoprazole 40 mg intravenously twice a day and a 7 day course of piperacillin-tazobactam.
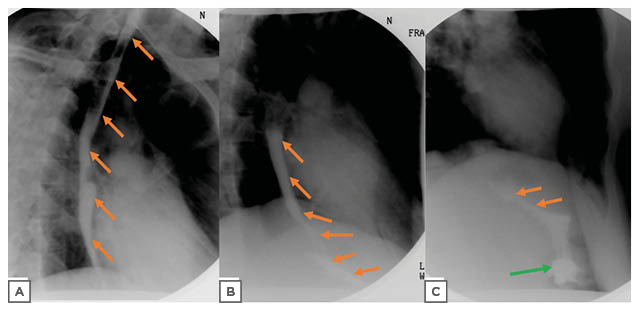
Figure 2: Barium esophagogram showed barium contrast proceeding from the upper part into the lower two parts of the oesophagus in A. In B, the barium is better visualised exiting the middle part and filling the lower part of the oesophagus. Subsequently, in C, the barium is seen exiting the third part of the oesophagus and moving past the gastroesophageal junction into the stomach. No contrast extravasation, intramural tracking, or penetration was detected. The orange arrows indicate the barium within the oesophagus and the green arrow indicates the location of the stomach.
After 7 days, total parental nutrition was discontinued and the patient was begun on a diet, starting with a clear liquids and then progressed to a regular diet, which was well tolerated. The patient was discharged from the hospital on Day 9. The patient did not experience any complications during the hospital course.
DISCUSSION
The diagnosis of BS was made in the emergency department based on the patient’s history of BS, the presenting symptom of vomiting, and CT findings of extensive mediastinal air tracking that was most pronounced around the lower third of the oesophagus. These findings were collectively highly suggestive of an oesophageal perforation.8 Although a site of oesophageal perforation was not confirmed with radiologic studies, CT imaging of the chest is not a sensitive modality to detect oesophageal perforation, with a 17% detection rate among patients with confirmed BS.8 In addition, there are other descriptions of CT imaging not identifying oesophageal pathology in BS patients.8-10 While contrast oesophagograms have a much higher sensitivity for oesophageal perforation than chest CT, the false negative rate is still as high as 10%.11 There are several reported cases of contrast oesophagograms using both gastrografin and barium in which the patient’s oesophageal perforation was not detected in BS patients.9-11 The patient reported vomiting, and radiologic studies revealed subcutaneous emphysema, which are two of the three components of Mackler’s triad, but lacked the third Mackler triad component of chest pain.12 While Mackler’s triad when present can be suggestive of BS, it is only present in 5% of patients with BS.12
Since the diagnosis of BS was not endoscopically confirmed due to a decision that the risks of the procedure outweighed the benefits, alveolar rupture remains on the differential. The most common causes of non-traumatic pneumomediastinum that have been observed to occur in the setting of vomiting are alveolar rupture and oesophageal perforation.13 Alveolar rupture can be identified with the Macklin effect, which is CT imaging that demonstrates gas travelling centrally along the perivascular and peribronchial interstitial sheaths into the mediastinum.14 The Macklin effect is found in 89–100% of alveolar rupture cases, making this a less likely diagnosis in this patient, since these findings were absent in this case.14 Other causes of pneumomediastinum include tracheobronchial injury, head and neck trauma, and abdominal injury, but the underlying conditions that would result in these aetiologies were not present in this patient.13
The decision to triage this patient to the Medicine team for conservative management initially triggered a discussion whether such a plan of care was sufficient. It was ultimately decided that the decision to conservatively manage the patient was appropriate, based on guidelines outlined in Cameron et al.,7 given that the patient had minimal symptoms, no evidence of neoplastic tissue, no evidence of obstruction, no detected intramural or transmural perforation, no pleural space contamination, a swallow study that showed no active leakage of contrast, and was at a hospital that has readily available cardiothoracic surgeons and access to a repeat contrast study at any time of the day.7,15 Conservatively managed patients that fit these criteria face an average mortality of 17% (range: 0–33%), comparable to the average mortality of 12% (range: 0–31%) for primary oesophageal repair.16 The current literature primarily recommends surgical management of patients with BS.17 However, there is a small but growing number of reports that describe situations in which conservative treatment has been used successfully. Between the years 1979–2021, there have been 95 reported cases of BS in which the patient survived the episode with completely conservative treatment without the need for any type of an invasive intervention.6,7,10,18-47 Identifying those who are appropriate for conservative management of BS could ultimately benefit such patients. So far, medically managed BS has been associated with significantly shorter hospitalisations and a lower cost of treatment.48
Following the decision to manage conservatively, constructing a treatment plan proved difficult, given the lack of formal guidelines on the subject and the subsequent variation in approaches to conservatively managed BS. Most of the available literature suggested at least a week of strict NPO, possible parenteral nutrition, 1–2 weeks of broad-spectrum antibiotics, and acid suppressive therapy.7,24,49,50 However, there was conflicting information regarding NPO duration, acid suppressive regimens, the use of parental nutrition, and the choice of antibiotic agent, as well as the role of chest tubes and endoscopy.
A recurrence of BS is extremely rare, with only 12 prior confirmed reports identified among adult patients as of December 2021.45,51-54 Among all 13 cases of recurrent BS in adult patients, there are several aspects of this case that make it unique. This is one of only two identified cases of recurrent BS among adult patients in which both occurrences were managed conservatively.43,44 Between these two cases, this case stands out because the episodes of BS were separated by 9 months, as compared with the other case in which the reoccurrence happened within several weeks.43,44 In addition, between these two cases, this is the first in which the patient denies having regular alcohol intake or other chronic risk factors.
While informal criteria exist to help guide clinicians to which BS patients may be appropriate candidates for conservative management, there is a lack of high-quality studies supporting these criteria. In addition, while there are certain aspects of conservative BS management that appeared frequently in prior case reports, ideal management of recurrent BS remains unclear based on the existing literature. The components of conservative management have varied significantly between different BS cases. This is likely due to the infrequency of BS. A prospective study would be an ideal next step to explore optimal management; however, this would likely be difficult to arrange due to the rarity of a patient appropriate for conservative BS management. For now, through continued reporting of conservatively managed BS cases, the variation of therapeutic approaches and their subsequent outcomes can be further explored, with the ultimate goal of identifying and standardising the optimal treatment regimen.







