Abstract
Helicobacter pylori infection is the most prevalent chronic bacterial infection in the world. The prevalence of H. pylori infection ranges from approximately 10–90% and is influenced by age, country, socioeconomic status, nutritional status, urbanisation, hygiene, and diagnostic tools available. In general, chronic H. pylori infection can lead to chronic antral gastritis, peptic ulcer disease, primary gastric lymphoma, and gastric adenocarcinoma. As public hygiene and sanitation have improved, the rates of H. pylori infection and related diseases have been declining annually in developed and rapidly developing countries, although the infection is still common in some geographic areas. In Taiwan, an Asian country with a high incidence rate of gastric malignancy, there is a similar trend of declining H. pylori prevalence rates. Prevalence rate differed vastly between rural and urban areas; however, rates have fallen greatly in recent decades. Optimal treatment of H. pylori infection in children has not yet been determined and will require further collaborative studies. However, eradication failures are concerning since global rates of antibiotic resistance are increasing and therapy for H. pylori infection is increasingly prescribed. In Taiwan, the overall antimicrobial resistant rates to clarithromycin, metronidazole, and levofloxacin were 23.4%, 20.3%, and 11.8%, respectively. With the propagation of public health education, advancement of diagnostic tools, and patient-specific tailoring of therapeutic strategies, the prevalence and eradication failure rate of H. pylori infection in children should improve in the near future, both in developed and developing countries.
INTRODUCTION
Helicobacter pylori infection is the most prevalent chronic bacterial infection in the world. The prevalence of H. pylori infection ranges from approximately 10–90% and is influenced by age, country, socioeconomic status, nutritional status, urbanisation, hygiene, and diagnostic tools available.1-3 H. pylori was discovered by Warren and Marshall in 1983.4 Infection is generally acquired in early childhood, primarily by mother–child and sibling–sibling transmission, and is usually life-long in the absence of effective treatment.5,6 Chronic H. pylori infection can lead to chronic antral gastritis, peptic ulcer disease, primary gastric lymphoma (most commonly mucosa-associated lymphoid tissue [also known as MALToma]), and gastric adenocarcinoma.7-9 More recently, it has been suggested that H. pylori may be associated with extra-intestinal disorders, including short stature, immune thrombocytopenic purpura, refractory iron deficiency anaemia (IDA), vitamin B12 deficiency, and eye, skin, respiratory, and psychiatric disorders.4,7,8 However, H. pylori infection may be beneficial for several diseases, such as asthma, obesity, and inflammatory bowel disease.8,10
Despite worldwide prevalence of chronic H. pylori infection, the route of transmission is still unclear. Interpersonal transmission appears to be the main route, although environmental transmission, such as drinking contaminated water, remains possible. Intrafamilial infection rather than community-acquired infection has also been proposed.5 In our previous study on H. pylori infection in children in northern Taiwan, we found no significant association between the patients’ blood type, age, sex, source of water supply, parents’ educational level, or the status of family members’ H. pylori seropositivity.11 It is therefore essential that we improve our understanding of H. pylori infection in children in Taiwan, an Asian country with a high incidence rate of gastric malignancy.
EPIDEMIOLOGY
The prevalence of H. pylori infection in children varies between 4.9% and 73.3% worldwide, depending on country, geographic area, target population, patient age, year of specimen collection, sample size, and detection methods used in the studies.5,12,13 As general public hygiene and sanitation improves, the rates of H. pylori infection and related diseases have been declining yearly in developed and rapidly developing countries, although the infection is still common in some geographic areas.14 According to the 1996 National Health and Nutrition Examination Survey in the USA, 24.8% of participants aged 6–19 years displayed evidence of H. pylori infection.15 However, the rate of infection in the 0–20-year age group decreased to 6.0% in a 2012 study carried out in Texas, USA.16 In Russia, the seroprevalence of H. pylori declined from 30.0% among children aged <5 years in 1995 to 2.0% 10 years later.17 Studies from Asian countries also have shown decreasing rates of H. pylori infection over the past 40–50 years.18 In Japan, the overall seroprevalence rate decreased from 72.7% in 1974 to 39.3% in 1994, which has led to a decline in the number of cases of gastric cancer in Japan.19 In a study including children from the Guangzhou province in southern China, the seroprevalence of H. pylori was found to significantly decrease from 62.5% in 1993 to 49.3% in 2003.20 Figure 1 summarises the global prevalence rates of H. pylori in children during recent decades.
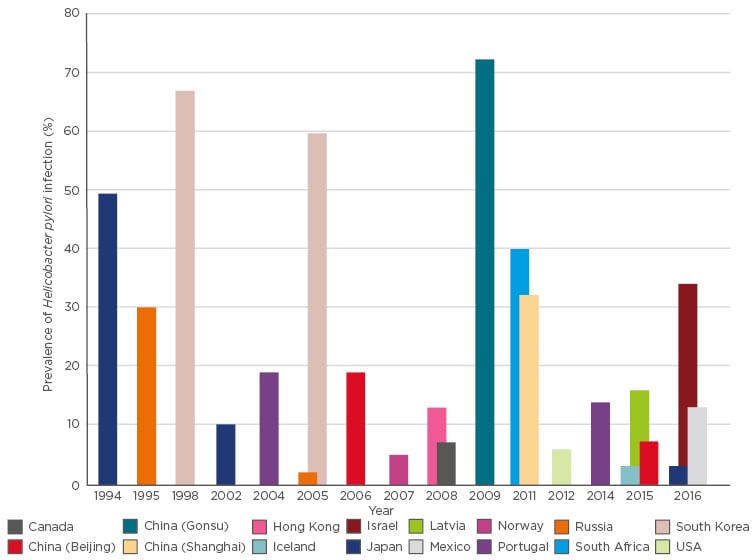
Figure 1: Global prevalence of Helicobacter pylori infection in children.
In Taiwan, several seroprevalence studies were performed in different districts in recent decades. We also found the similar trend of declining prevalence rate; Figure 2 shows the seroprevalence rate in different regions in Taiwan over the last 20 years. The prevalence rate differed greatly between rural and urban areas and has fallen dramatically in recent decades. In a study performed in two rural islands of Taiwan in 2005, Chen et al.21 reported that the seroprevalence of H. pylori among the randomly selected school-aged children (13–15 years) on Green Island and Lanyu Island were 82.4% and 71.0%, respectively (Figure 2). In 2008, Chi et al.22 also reported that the H. pylori infection rate based on carbon-13 urea breath test in Lanyu Island was 54.7% in secondary school students. There was a decline in the prevalence rate in this isolated and closed island following the introduction of public health education regarding proper sanitation and food handling. However, the rate was still high when compared to that of mainland Taiwan.
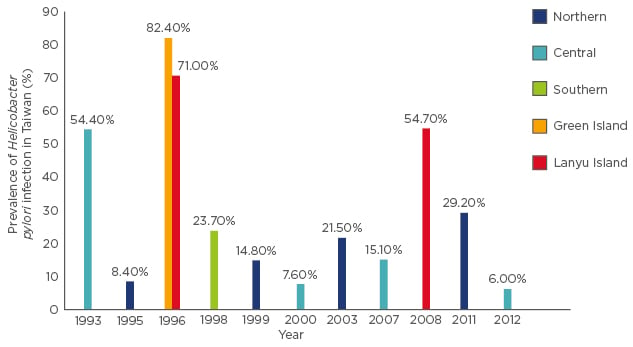
Figure 2: Prevalence of paediatric Helicobacter pylori infection in Taiwan.
In a study in rural central Taiwan in 2015, Wu et al.23 found that the overall H. pylori infection rates were 6.0% in children between 0 and 15 years of age, much lower compared to previous studies of the same region. In our previous study in northern urban Taiwan in 2003, the seroprevalence of H. pylori in primary school students, aged 7–12 years, was 21.5%, which was higher than that reported in previous Taiwanese reports.11 Chiu et al.24 also found that the total prevalence of H. pylori infection in children aged 4–18 years was 29.2% in northern urban Taiwan in 2011; however, the age-specific seroprevalence of H. pylori infection in Taiwan was 40.9% for the 10–19 year age group in a nationwide study.25 Age and urbanisation were found to be dependent factors for the variations in prevalence rates found in different studies (Figure 2).
CLINICAL MANIFESTATIONS
Gastritis and Peptic Ulcer Diseases
While infection with H. pylori infection is common, the spectrum of the disease is wide and complex; it is associated with many disorders, most strongly with chronic gastritis and peptic ulcers.26 H. pylori infection is also associated with chronic antral gastritis, which is related to duodenal ulcer, gastric ulcer, and gastric carcinoma. Many endoscopic studies have supported the association between H. pylori infection and gastroduodenal pathology in children.27
A pooled analysis of early reports demonstrated that the rate of antral gastritis for children with H. pylori infection (compared with uninfected subjects) ranged from 1.9–71.0%.28 The prevalence of H. pylori in children with duodenal ulcers was high compared with children with gastric ulcers.28 A subsequent retrospective study from Japan confirmed that the prevalence of H. pylori was very high in antral gastritis and duodenal ulcer patients (98.5% and 83.0%, respectively). In another retrospective review of 619 Chinese children who had undergone upper endoscopy for investigation of upper gastrointestinal symptoms, Tam et al.29 found that 6.9% had a peptic ulcer; the prevalence of H. pylori infection was present in 56.8% of duodenal ulcer and 33.3% of gastric ulcer patients.
In a study from southern Taiwan, Huang et al.30 reported 47.7% of childhood peptic ulcer disease cases had H. pylori infection, and 16.5% of the children reported previous use of non-steroidal anti-inflammatory drugs (NSAID). Children with H. pylori-related peptic ulcer disease had a significantly higher mean age, antral chronic inflammatory score, rate of familial peptic ulcer disease, and presence of duodenal ulcer and nodular gastritis than those with NSAID-related and non-H. pylori, non-NSAID-related peptic ulcer diseases.
However, there have been conflicting results of studies looking for an association between infection with H. pylori and peptic diseases in children. In a prospective European multicentre pilot study on the incidence of gastric and duodenal ulcer disease in children, Kalach et al.31 found that ulcers occurred in only 10.6% of cases, with H. pylori infection reported in only 26.7% of these. In our recent study performed in northern Taiwan, 56.8% of children with H. pylori infection were completely asymptomatic, and the remaining 45.2% had at least one gastrointestinal symptom.24 Our result supported the hypothesis that the abdominal complaints found in children with H. pylori infection were not caused by the infection itself.
Recurrent Abdominal Pain
The relationship between recurrent abdominal pain (RAP) in childhood and H. pylori infection is still not clear;28 the results of two uncontrolled trials have suggested improvement of clinical symptoms after H. pylori infection eradication.32,33 In a meta-analysis of 38 studies between 1966 and 2009, Spee et al.34 found no association between RAP and H. pylori infection in children. In a study in Icelandic children, any abdominal pain in the previous 6 months was reported by 36% of H. pylori-negative children and 43% of H. pylori-positive children.6 This was a considerably lower prevalence than that reported in other studies, including in a Swedish study where RAP was recorded at 13%, and any abdominal pain was reported by 63% and 70% of children in Sweden35 and Germany,36 respectively. Large-scale multicentre trials performed in children are required to uncover whether a connection exists between H. pylori infection and RAP.
Gastro-oesophageal Reflux Disease
Recently Daugule et al.37 showed a significantly higher prevalence of H. pylori among patients with reflux oesophagitis compared to patients with hyperaemic gastropathy alone. The prevalence of reflux oesophagitis was 14.2% among H. pylori-positive and 3.3% among H. pylori-negative patients with an odds ratio of 5.5 for H. pylori infection among children with reflux oesophagitis. However, H. pylori has been found to be inversely correlated with the prevalence of gastro- oesophageal reflux disease, and certain studies have shown aggravation of oesophagitis after eradication.7 A Hong Kong study found that H. pylori eradication was the only predictor of gastro-oesophageal treatment failure.38 They also showed H. pylori eradication increased oesophageal acid exposure and might adversely affect the clinical course of reflux disease in a subset of patients.39
Iron Deficiency Anaemia
Over the past two decades, an association between H. pylori and paediatric IDA has been established.8 However, the issues of whether paediatric H. pylori infection is linked causally to IDA and whether treatment or resolution of H. pylori infection would improve iron stores in children are still matters of great debate. It is often difficult to distinguish between the confounding effects of poverty, poor nutrition, and poor compliance with treatment when examining the relationship between H. pylori and anaemia. While studies have shown that eradication of H. pylori in children with refractory anaemia results in improved haematologic indices in the short term, no study, until now, had sufficient follow-up time to determine if there is a recurrence of anaemia after treatment.
One explanation for the relationship between H. pylori infection and IDA involves the effect of H. pylori gastritis on gastric acid secretion and iron absorption.8 Non-haem iron accounts for 80% of dietary iron in industrialised countries,40 and hydrochloric acid in acid secretions is crucial to the effective absorption of non-haem iron. In a study from Taiwan, Huang et al.41 reported 31.7% adolescents with traceable aetiologies of iron deficiency were infected by H. pylori. With only 38.5% of occult blood tests of patients’ stool testing positive, they found that H. pylori infection resulting in iron deficiency or IDA mainly influenced iron absorption versus bleeding. Eradication of H. pylori could be considered in cases that were refractory to iron supplementation and in the case of frequent relapses, assuming that other causes, such as coeliac disease and inflammatory bowel disease, have been excluded. Randomised, double-blind, and placebo-controlled trials of sufficient size and power should evaluate the long-term effect of H. pylori eradication in children with IDA. In children with refractory IDA, endoscopic examination may be necessary to rule out not only the presence of H. pylori but other aetiologies of anaemia.
Short Stature and Failure to Thrive
The available evidence regarding H. pylori infection and its effect on growth in children is also controversial.8 Some cross-sectional analyses have indicated that H. pylori-infected children had subnormal growth retardation compared with non-infected children, but other studies did not support such findings.8,27 Growth and failure to thrive (FTT) are important, complex issues, and, thus, assessing the association between H. pylori infection and growth is highly intricate. Some studies report that H. pylori may cause FTT, but the evidence is conflicting.27
Perri et al.42 suggested that H. pylori infection is associated with growth delay in older Italian children, poor socioeconomic conditions, and household overcrowding. These findings are consistent with the hypothesis that H. pylori infection is one of the environmental factors capable of affecting growth. Based on the data of an observation cohort in southern Taiwan, Yang et al.43 reported the H. pylori-infected children had significantly lower body weight (BW) and BMI than sex and age-matched controls at enrolment. After 1-year follow-up, the H. pylori-infected children had profoundly lower BW, BMI, and net BW gain than the non-infected children.43 They also found H. pylori infection could be associated with decreased serum acylated ghrelin levels, BW, and height in children. Successful H. pylori eradication could restore ghrelin levels and the increase of BW and height in the infected children with growth retardation.44
However, in our recent study in northern Taiwan, we compared the H. pylori infection status in children with FTT and a matched control group.24 There was no significant difference between infected and uninfected children with regard to BW, height, and BMI, and no association between FTT and H. pylori infection. Therefore, physicians should not overtreat H. pylori-infected children with FTT; the decision for eradication should be individualised and evaluated carefully.
In another cross-sectional study of Turkish children aged 4–16 years who underwent upper gastrointestinal endoscopy for RAP and dyspeptic complaints, Süoglu et al.45 found that the effect of H. pylori infection on mean standard deviation scores of height for age was statistically insignificant after correction for breastfeeding, IDA, and socioeconomic level. After controlling for sociodemographic variables, the authors did not find significant differences in the nutritional parameters between infected and uninfected children. Taken together, the results of these studies that point to the presence or absence of an association between H. pylori and growth are subject to potential limitations. We conclude that future work in this area is needed to elucidate the importance of these factors.
ASTHMA, INFLAMMATORY BOWEL DISEASE, AND OTHERS
H. pylori infection does not always have negative consequences and some effects may be beneficial. A previous cross-sectional study revealed that adults <40 years of age with an H. pylori infection had an inverse correlation with asthma.15 In addition, a beneficial relationship between H. pylori infection and other atopic diseases, obesity, and inflammatory bowel disease has also been reported.16,46 Therefore, the decision to screen and eradicate H. pylori infection in children should be justified and individualised.
ANTIBIOTIC RESISTANCE
The current recommended first-line H. pylori eradication regimen is a clarithromycin-based triple therapy, including a proton pump inhibitor, and either amoxicillin or metronidazole. Such a regimen achieved an eradication rate of approximately 70–85% in the early 1990s;5,47 however, eradication failures are concerning at the present time and they are likely to be more critical in the future, given that global rates of antibiotic resistance are increasing and therapy for H. pylori infection is increasingly prescribed.5 This is reflected in the most recent Maastricht Recommendations,48 which state that susceptibility testing should be performed prior to therapy in regions with high clarithromycin resistance rates. Therefore, the consensus recommended that clarithromycin- based triple therapy should not be used empirically in the first-line therapy, when the local resistance rate is >15–20%.48
Although surveillance of antimicrobial-resistant H. pylori in each domestic area should be recommended and warranted, especially for clarithromycin and the commonly applied amoxicillin and metronidazole, only a few laboratories routinely grow H. pylori from biopsy specimens and perform antibiotic susceptibility testing on the isolates. For this reason, data on antibiotic resistance of H. pylori in children are sparse for many countries and geographic areas. The prevalence of bacterial antibiotic resistance is regionally variable and appears to be markedly increasing with time in many countries. In a study from Germany, overall resistance of H. pylori isolates, obtained from children and adolescents, to metronidazole, clarithromycin, and rifampicin, were 28.7%, 23.2%, and 13.3%, respectively, while resistance to amoxicillin was rare (0.8%).49 Simultaneous resistance to metronidazole and clarithromycin was observed in 7.7% of the isolates and 2.3% were resistant to metronidazole, clarithromycin, and rifampicin. In Figure 3, global antibiotic resistant rates of H. pylori in children are shown.
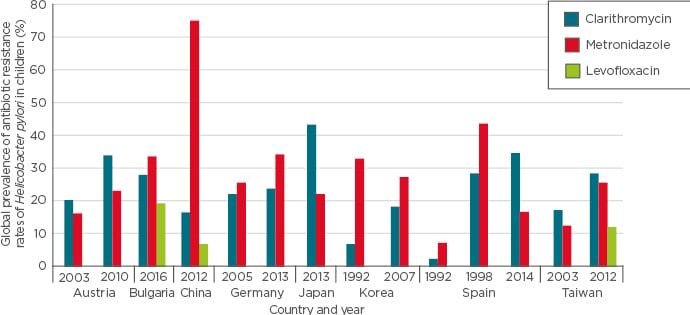
Figure 3: Global prevalence of antibiotic resistance rates of Helicobacter pylori in children.
In Taiwan, Lu et al.50 recently investigated the changing antimicrobial susceptibility of H. pylori isolated from children over the past two decades. Conventional bacterial cultures were performed as the diagnostic standard. Minimal inhibitory concentrations of antibiotics were determined by an Epsilometer test. The overall antimicrobial resistant rates to clarithromycin, metronidazole, and levofloxacin were 23.4%, 20.3%, and 11.8%, respectively;50 there was no recorded resistance to amoxicillin. In comparison, regarding the resistance rates of clarithromycin and metronidazole in the periods of 1998–2007 and 2008–2016, isolates in the latter period had higher resistance rates of clarithromycin (28.6% versus 17.2%) and metronidazole (25.7% versus 13.8%). The rates of resistance to clarithromycin and metronidazole have increased compared to levels a decade ago and this rapid evolution of antimicrobial resistance of H. pylori in children has led us to be more conscientious in the treatment of childhood H. pylori infection (Figure 3).
Due to the clinical importance of decreased eradication rates, regional variation in antibiotic resistance rates, and the marked increase over time of antibiotic resistance rates in the past decades, there is a critical need for determination of current rates at a local scale, and also in individual patients. Patient-specific tailoring of effective antibiotic treatment strategies may lead to reduced treatment failures and less antibiotic resistance.
CONCLUSION
As public hygiene and sanitation improves, the prevalence of H. pylori infection in children is declining in developed countries and clinical paediatricians need to re-evaluate when to test for H. pylori and whether to treat the infection.29 However, we must be aware that the prevalence of H. pylori infection is still high in most countries worldwide, especially in developing countries, and optimal treatment of H. pylori infection in children has not yet been determined and will require further collaborative studies. Various regimens, including a proton pump inhibitor, have been used for treatment of H. pylori infection in adults,5 but few studies concerning the clinical features, efficacy, and safety of treatment have been carried out in children. H. pylori treatment for children and adolescents seems safe, but further surveys evaluating adverse events need to be conducted.
With the propagation of public health education, advancement of diagnostic tools, and patient-specific tailoring therapeutic strategies, the prevalence and eradication failure rate of H. pylori infection in children should decrease in the near future, both in developed and developing countries.








