Abstract
Background: The anti-TNF drugs adalimumab (ADA) and infliximab (IFX) are effective treatments for inflammatory bowel disease (IBD). However, 40% of patients lose response, often due to the development of antibodies-to-ADA (ATA) and antibodies-to-IFX (ATI). While low ATA/ATI titres (<200 ng/mL) are associated with better outcomes and high ATA/ATI titres (>1,000 ng/mL) are associated with poorer outcomes, the significance of intermediate ATA/ATI titres (200–999 ng/mL) is not well understood. This study aims to investigate the impact of intermediate ATA/ATI titres on outcomes in patients with IBD.
Methods: A retrospective chart review of 376 patients with IBD was conducted. The primary clinical outcome was persistence on anti-TNF therapy for 1 year after the measurement of ATA/ATI titres. The participants consisted of patients with IBD treated with IFX or ADA at the University of Maryland Medical Center’s Inflammatory Bowel Disease Program between October 2016 and October 2019.
Results: Out of 322 patients with low titres, 271 persisted on their original anti-TNF, compared with nine out the 15 patients with intermediate titres (p=0.026) and one out the 10 patients with high titres (p<0.0001). The odds ratio of persistence when comparing intermediate titres to low titres was 0.26 (0.09–0.80), and when comparing high titres to low titres was 0.02 (0.00–0.14).
Conclusion: Patients with intermediate titres were more likely to lose response to anti-TNF drugs and require a change in anti-TNF therapy than patients with low titres. Although the sample size of patients with intermediate titres was small, providers should consider dose optimisation of anti-TNF drugs, with or without the addition of an immunosuppressant, when intermediate titres are present.
INTRODUCTION
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is a chronic autoimmune condition that involves the intestines.1 IBD is characterised by chronic inflammation of the gastrointestinal tract, the pathogenesis of which involves an increase in the inflammatory cytokine TNF-α. This makes anti-TNF-α biologics such as adalimumab (ADA) and infliximab (IFX) effective options for the treatment of IBD.2-5 Of all patients given ADA and IFX, about 30% are primary non-responders, meaning that they do not respond to treatment initially. Another 40% of patients lose response over time, meaning that they do respond to the treatment initially, but symptoms gradually return. One of the causes of loss of response is the development of antidrug antibodies.6-11 This is a concern because most patients with IBD require long-term treatment and, with a limited number of treatments available, it is crucial to ensure that these drugs remain effective for as long as possible.
One of the ways that providers can optimise the treatment plan for a patient with IBD and prolong the benefits of a given biologic therapy like ADA or IFX is through therapeutic drug monitoring (TDM).12-23 TDM involves taking routine measurements of the drug and antidrug antibody levels in a patient with IBD to ensure that the drug levels are therapeutic and the antidrug antibody titres are low or undetectable. While it is known that low antidrug antibody titres (<200 ng/mL) are associated with therapeutic ADA and IFX concentrations and better clinical outcomes,5,24-32 and high antidrug antibody titres (>1,000 ng/mL) are associated with subtherapeutic ADA and IFX concentrations and poorer clinical outcomes,6 the significance of intermediate antidrug antibody titres (200–999 ng/mL) are currently not well understood. At the authors’ centre, the interpretation of antidrug antibody titres is at the discretion of the ordering provider. While there is no standard protocol in place, generally patients with low antidrug antibody titres are followed clinically for recurrent symptoms, such as signs of inflammation, and patients with high antidrug antibody titres are switched to a different anti-TNF drug or novel biologic, sometimes with the addition of an immunosuppressant when few therapeutic options remain. However, the approach to patients with intermediate antidrug antibody titres is unclear.
This study aims to address the gap in knowledge around intermediate antidrug antibody titres in order to give providers better guidance for managing patient treatments through TDM.
METHODS
This paper outlines a retrospective cohort study that took place at the University of Maryland Medical Center’s Inflammatory Bowel Disease Program. The study participants consisted of patients with either Crohn’s disease or ulcerative colitis who were being treated with ADA or IFX between 15th October 2016 and 15th October 2019 (Figure 1). The participants had at least one measurement of antibodies-to-ADA (ATA)/antibodies-to-IFX (ATI) and ADA/IFX during the study time period, with all assays done using LabCorp software (Laboratory Corporation of America Holdings, Burlington, North Carolina, USA) for comparability. Of the 376 patients identified, 157 patients were taking ADA and 219 patients were taking IFX. Of the 157 patients taking ADA, 113 had serial measurements and 44 had singlet measurements. Of the 219 patients taking IFX, 171 had serial measurements and 48 had singlet measurements.
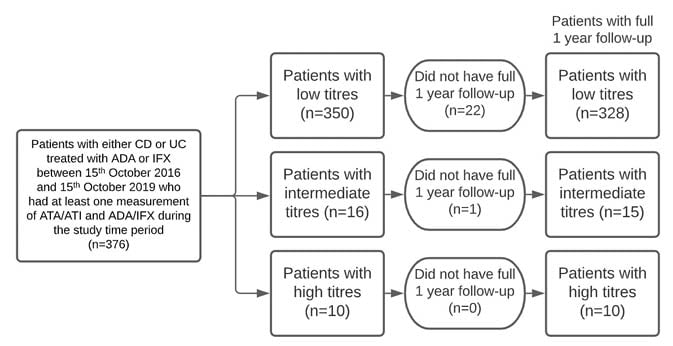
Figure 1: Schematic flow diagram of patient enrolment and analyses.
ADA: adalimumab; ATA: antibodies-to-adalimumab; ATI: antibodies-to-infliximab; CD: Crohn’s disease; IFX: infliximab; UC: ulcerative colitis.
The primary exposure variable examined was the patient’s ATA/ATI titres, with thresholds as follows: low titres: <200 ng/mL; intermediate titres: 200–999 ng/mL; and high titres: ≥1,000 ng/mL.
The primary clinical outcome of interest was persistence on anti-TNF therapy for 1 year after the measurement of the ATA/ATI titres. Secondary clinical outcomes included the clinical response to therapy 1 year after measurement of ATA/ATI titres, the development of high ATA/ATI titres 1 year after measurement, the initiation of steroids within the 1-year study period, and a change in therapy made by the provider in response to the initial ATA/ATI titre measurement. Clinical response to therapy 1 year after the measurement of ATA/ATI titres was categorised into three groups: no response or worsening, partial response, and complete response, according to the physician’s global assessment of disease activity. Additional sub-analyses evaluated the effect of immunosuppressant use on the development of high ATA/ATI titres and clinical response. These clinical response rates were also categorised into three groups: no response or worsening, partial response, and complete response, according to the physician’s global assessment of disease activity.
Groups were compared using parametric or non-parametric statistical analyses as appropriate. Baseline characteristics were described by the mean and standard deviation for continuous variables and by the number and percentage within the group for categorical variables. Comparisons of these variables were made using Student’s t-test, chi-square test of homogeneity, or Fisher’s exact test based on the type of variable and the normality of the data. A p value of 0.05 was considered significant for differences between groups. A final logistic regression analysis was performed to examine the relationship between exposure group and persistence on therapy after adjustment for confounding variables. Patients without 1 year of follow-up were excluded from the tables below.
RESULTS
In this study, 376 patients were identified (patients taking ADA: 157; patients taking IFX: 219): 350 patients with low titres, 16 with intermediate, and 10 with high titres. Participant baseline characteristics such as age, smoking history, and extraintestinal manifestations were similar. However, patients with intermediate (five out of 16) and high (two out of 10) titre antibodies were more likely to have a family history of IBD than those with low titre antibodies (35 out of 350; Table 1). Of the original set of patients, 22 of 350 low titre, one of 16 intermediate titre, and zero of 10 patients with high titres did not have a full 1 year of follow-up and were, therefore, excluded from subsequent analyses.
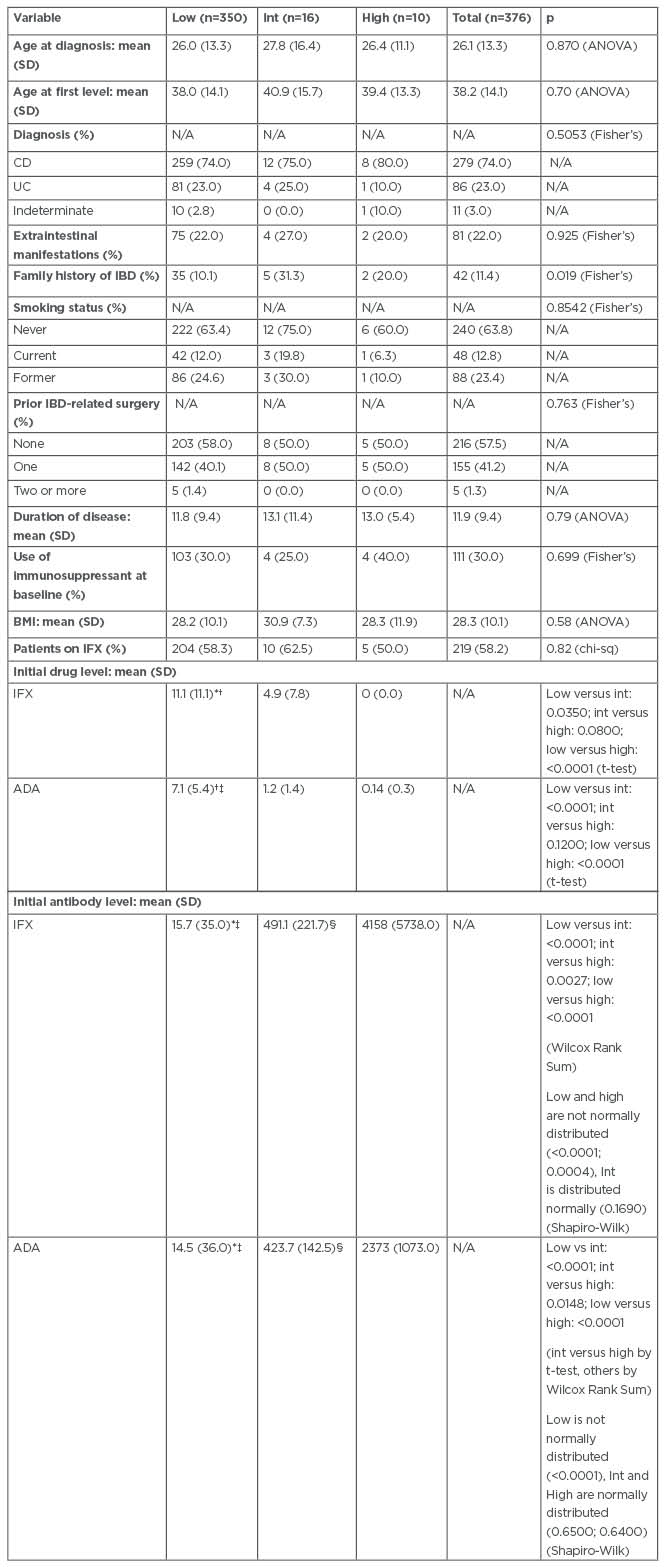
Table 1: Demographic and clinical characteristics of patients with inflammatory bowel disease who were treated with infliximab or adalimumab between 2016 and 2019 at the University of Maryland Medical Center’s Inflammatory Bowel Disease Program.
*Low titre group significantly different from intermediate titre group (p<0.05).
†Low titre group significantly different from high titre group (p<0.0001).
‡Low titre group significantly different from intermediate titre group (p<0.0001).
§Intermediate titre group significantly different from high titre group (p<0.05).
ADA: adalimumab; ANOVA: analysis of variance; CD: Crohn’s disease; chi-sq: chi-square test; Fisher’s: Fisher’s exact test; IBD: inflammatory bowel disease; IFX: infliximab; Int: Intermediate; N/A: not applicable; SD: standard deviation; Shapiro-Wilk: Shapiro-Wilk test; t-test: Student’s t-test; UC: ulcerative colitis; Wilcox Rank Sum: Wilcoxon Rank Sum test.
Persistence on Original Anti-TNF
It was found that 271 out of 322 (84%) patients with low titres persisted on their original anti-TNF treatment compared with nine out of 15 (60%) patients with intermediate titres and one out of 10 (10%) patients with high titres.
The odds ratio (OR) of persistence to original anti-TNF treatment when comparing intermediate titre to low is 0.280 (95% confidence interval [CI]: 0.096–0.827); when comparing high titre to low is 0.021 (95% CI: 0.003–0.169); and when comparing intermediate titre to high is 0.074 (95% CI: 0.007–0.746). Controlling for family history, the OR of persistence to original anti-TNF treatment when comparing intermediate titre to low is 0.260 (95% CI: 0.085–0.797), and when comparing high titre to low is 0.017 (95% CI: 0.002–0.143). When controlling for the above factors, the OR comparing high titre to intermediate was not significant (OR: 0.004; CI: <0.001–1.860; p=0.0785). In addition to the patients who did not have a full 1 year of follow-up, six out of 328 patients with low titres did not have data for their persistence on original anti-TNF treatment at 1 year and were, therefore, also excluded from this analysis.
Clinical Response to Therapy
In the low titre group, 45, 31, and 234 patients had no, partial, or complete response to therapy, respectively. In the intermediate titre group, six, zero, and eight patients had no, partial, or complete response, respectively; and in the high titre group, three, one, and three patients had no, partial, or complete response, respectively. The remaining patients with high titres did not have data at 1 year. The difference in the distribution of patients who showed no, partial, and complete responses was significantly different between the patients with low titres and intermediate titres (p=0.019), but not significantly different between patients with intermediate titres and high titres (p=0.440). There was a trend towards higher response rates in the patients with low titres compared with the high titres (p=0.061; Table 2). In addition to patients who did not have a full 1 year of follow-up, 18 out of 328 patients with low titres, one out of 15 patients with intermediate titres, and three out of 10 patients with high titres were excluded from the analysis because they did not have data for their clinical response at 1 year.
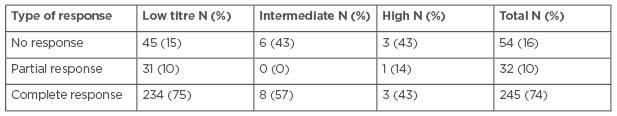
Table 2: Responses to anti-TNF therapy at 1 year in patients with inflammatory bowel disease who were treated with infliximab or adalimumab between 2016 and 2019 at the University of Maryland Medical Center’s Inflammatory Bowel Disease Program.
Note: Patients with missing data for this outcome were excluded from analysis.
It was found that 30, 19, and 161 out of 210 patients with low titres not taking immunosuppressants at baseline and 15, 11, and 72 out of 98 patients with low titres taking immunosuppressants at baseline had no response, partial response, or complete response to therapy, respectively (p=0.76). The clinical response rates in patients with initially low antibody titres with and without baseline immunosuppressant use were not significantly different. Similarly, in patients with low titres, baseline use of immunosuppressants did not change the risk of persisting on initial anti-TNF compared to those who did not use immunosuppressants at baseline.
Furthermore, it was found that four, zero, and seven out of 11 patients with intermediate titres not taking immunosuppressants at baseline and two, zero, and one out of three patients with intermediate titres taking immunosuppressants at baseline had no response, partial response, or complete response to therapy, respectively (p=0.54). The clinical response rates in patients with intermediate titres with and without baseline immunosuppressant use were not significantly different. In patients with intermediate titres, baseline use of immunosuppressants also did not change the risk of persisting on the initial anti-TNF therapy compared with those who did not use immunosuppressants at baseline.
Risk of Developing High-Titre Antibodies
In this study, six out of 324 patients with low titres developed high ATA/ATI titres, compared with three out of 15 patients with intermediate titres (p=0.005). In addition to patients who did not have a full 1 year of follow-up, four out of 328 patients with low titres were excluded from the analysis because they did not have data for the development of high-titre antibodies at 1 year.
Of the 13 patients with intermediate titres who had subsequent drug levels, five patients had their original anti-TNF dose increased in response to their initial measurement. Of these five patients, three went into remission and saw a drop in ATA/ATI titre from intermediate to low, while two showed no change or worsening of symptoms. Moreover, four of the 13 patients had their original anti-TNF dose increased and were started on an immunosuppressant in response to their initial measurement. Of these four patients, three went into remission and saw a drop in ATA/ATI titre from intermediate to low, while one showed improvement in symptoms. However, three of these 13 patients had no change to their therapy in response to their initial measurement. Of these, two went into remission and one showed no change or worsening of symptoms and saw an increase in ATA/ATI titre from intermediate to high. One of the 13 patients was switched to a different anti-TNF treatment in response to their initial measurement but was lost to follow-up.
Additionally, three out of 220 patients with low titres not taking immunosuppressants and three out of 102 patients with low titres taking immunosuppressants developed high titre antibodies (p=0.39). Of the intermediate titre patients, two out of 9 patients not taking immunosuppressants and one out of 4 patients taking immunosuppressants developed high titre antibodies (p>0.99).
Impact of Antibody Titre on Management
It was found that 25 out of 325 patients with low titres started steroids due to IBD, compared with three out of 15 patients with intermediate titres (p=0.12). Patients with high titres are not included here because none of the 10 patients started steroids within the study period, and six of these 10 patients were already on a steroid regimen prior to TDM.
The distribution of change in therapy made in response to the initial ATA/ATI titre levels is significantly different between the patients with low, intermediate, and high titres: 142 out of 325 patients with low titres, one out of 15 patients with intermediate titres, and one out of 10 patients with high titres had no change in their therapy in response to the initial titre measurement. Furthermore, 52 out of 325 patients with low titres, six out of 15 patients with intermediate titres, and nine out of 10 patients with high titres changed or stopped their current anti-TNF treatment in response to the initial titre measurement (Table 3).
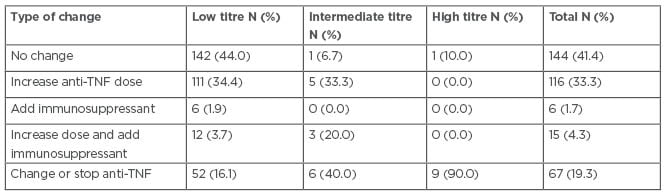
Table 3: Changes in therapy made in response to initial antibodies-to-adalimumab or antibodies-to-infliximab titres in patients with inflammatory bowel disease who were treated with infliximab or adalimumab between 2016 and 2019 at the University of Maryland Medical Center’s Inflammatory Bowel Disease Program.
Note: Patients with missing data for this outcome were excluded from analysis.
DISCUSSION
The data from this study indicate that the proportion of patients with intermediate titres who persist on their original anti-TNF therapy is lower than for patients with low titres. Clinical response rates seen in the patients with intermediate titres were different from the clinical response rates seen in the patients with low titres, but not different from the clinical response rates seen in the patients with high titres, suggesting that patients with intermediate titres have clinical response rates that more closely resemble those seen in patients with high titres. Patients with intermediate titres were also more likely to develop high ATA/ATI titres than patients with low titres. Altogether, these results suggest that patients with intermediate titres were more likely than patients with low titres to develop high ATA/ATI titres, lose response to anti-TNF treatment, thus requiring a change to anti-TNF therapy, and more closely resemble clinical response rates seen in patients with high titres. Thus, the identification of intermediate titre antidrug antibodies is a poor prognostic sign that warrants further intervention, which could entail a repeat assessment of antidrug antibodies before a subsequent infusion or injection, dose escalation, addition of an immunosuppressant, or both raising the dose and adding an immunosuppressant (Figure 2).
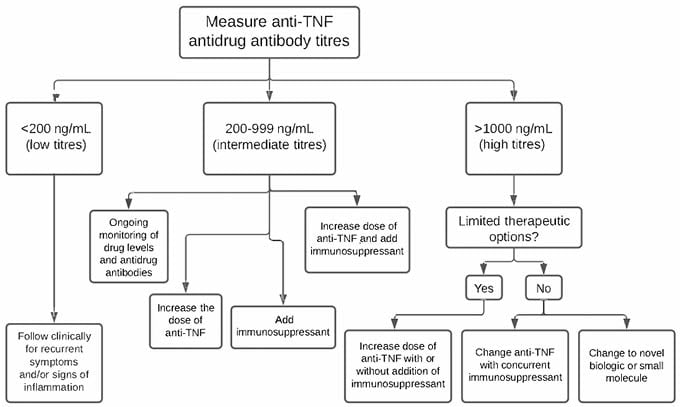
Figure 2: Clinical decision tree for patients with inflammatory bowel disease based on their antidrug antibody titre.
The finding from this study that patients with low titres persist more than patients with high titres on their original anti-TNF therapy is consistent with de Boer et al.,13 who found that patients with high titres frequently require switching to an alternative anti-TNF.7 Although there was not quite a statistically significant difference in the distribution of response to therapy between patients with low titres and patients with high titres, there were more patients with low titres with complete response (251 out of 331) compared with patients with high titres with complete response (three out of seven), and fewer patients with low titres with no response (47 out of 331) compared with patients with high titres with no response (three out of seven). It was found that low ATA/ATI titres are associated with better clinical outcomes, such as a complete response, whereas high ATA/ATI titres are associated with poorer clinical outcomes, such as no response. These findings are consistent with Mazor et al.,25 who found that high ATA titres are associated with disease activity.8 In the management of rising ATA/ATI production, studies by both Ben-Horin et al.22 and Vermeire et al.23 found that concomitant immunosuppressive therapy suppresses the formation of ATI and restores clinical response in patients with IBD.9,10 However, in the study outlined here, baseline immunosuppressant use did not seem to have a significant effect on the number of patients who went on to develop high ATA/ATI titres or the clinical response of patients. It is not clear whether adding an immunosuppressant at the time of identification of intermediate titres would have improved outcomes.
There are several strengths and few limitations in this study. The strengths include the overall sample size, the use of a single assay to measure IFX and ADA levels and antidrug antibodies, and carrying out adjusted analyses. A weakness of the study was its retrospective nature; nonetheless, most of the variables were collected with few cases of missing data. Additionally, as the University of Maryland is a referral centre for IBD care, these results may not be generalisable to the community at large. Lastly, a relatively small number of patients with intermediate antidrug antibodies participated, so the study was likely underpowered to identify small to moderate differences between the groups. This also limits the ability to study the specific interventions in response to antidrug antibody levels from this data. Nevertheless, to the author’s knowledge, this is the largest study of intermediate antidrug antibody levels in the literature.
CONCLUSION
Patients with intermediate titres are more likely than patients with low titres to develop high ATA/ATI titres, lose response to anti-TNF, and require a change in anti-TNF therapy, meaning that these patients more closely resemble the clinical response rates seen in high titre patients. However, the authors suspect that the ‘intermediate’ antidrug antibody titres are not one monolithic group, and that there are likely to be more precise ranges of titres that put patients at a higher or lower risk of developing high antidrug antibodies titres later on.
Although the sample size of patients with intermediate titres in this study was small, based on these findings the authors suggest that patients with intermediate antidrug antibody titres undergo active changes in treatment. For example, providers should consider a dose escalation of IFX or ADA, with or without concurrent immunosuppressant repeat drug and antidrug antibody levels, to assess for rising titres.
A follow-up study with a greater sample size of patients with intermediate titres should be considered in order to strengthen the association that this study found between intermediate ATA/ATI titres and an increased risk of developing high ATA/ATI titres and a subsequent loss of response to treatment. Baseline immunosuppressant use did not impact the development of intermediate titre antidrug antibodies. Future studies should also stratify patients based on baseline immunosuppressant use and determine whether adding immunosuppressants in patients with intermediate titres reduces antidrug antibody titres and prevents a loss of response.








