Meeting Summary
At the 14th Annual Congress of European Crohn’s and Colitis Organisation (ECCO), a symposium was convened to discuss the present and future of personalised care for patients with inflammatory bowel disease (IBD). IBD is not one disease: the clinical presentation, disease course, and treatment response differ in every patient. As such, personalised care is considered the best approach for effective management.
Importantly, it is acknowledged that IBD is not confined to the gut. Although the predominant symptoms manifest in the organ, the inflammation is likely to be systemic. The importance of learning from and collaborating with specialists who treat associated conditions, such as spondyloarthritis (SpA), will become the key to managing IBD at the individual level.
IBD is known to be influenced by genetic as well as environmental factors; however, some are yet to be identified. Advances in understanding ‘omes’ (e.g., genome, transcriptome, microbiome, etc.) and how they impact a person’s IBD journey are rapidly occurring. At the congress, experts provided their insights into recent developments in personalised care and how to optimise current tools at their disposal, as well as evolving methodologies that are anticipated to offer increased efficiency in the future, e.g., the introduction of systems biology.
The Myth of the Typical Inflammatory Bowel Disease Patient
Doctor Peter Irving
IBD is not one disease, but, rather, a whole range of diseases. It is a systemic immune disorder; therefore, in addition to causing conventional gastrointestinal symptoms, it also gives rise to inflammation outside of the gut. Extraintestinal manifestations include inflammation in the joints, skin, and eyes. Furthermore, IBD also conveys an increased risk of venous thromboembolism and arterial disease including ischaemic heart disease, cerebral vascular disease, and peripheral
arterial disease.1
Because every individual has a unique phenotype, IBD is not predictable. This gives rise to different disease courses and varying disease severity. Transcriptomics may help to define phenotypes, but this inherently increases the complexity of diagnosis and treatment. Different phenotypes may also suggest different treatment goals both within and between patients, but perhaps the greatest difficulty lies in choosing between the available treatment options to have the best chance of achieving these goals.
Inflammatory Bowel Disease Subgroups: The Secret of Hitting the Mark
Professor Jonas Halfvarson
Historically, as a consequence of the restrictions of conventional therapies, the goal of treatment was to improve symptoms. Over time, this goal has evolved from treating symptoms to controlling inflammation to achieving remission. A major breakthrough occurred with the introduction of targeted therapies or ‘biologics’, including TNFα inhibitors (anti-TNFα) and more recently, IL-12/IL-23 inhibitors, anti-integrins, and JAK inhibitors. The advent of targeted therapies improved endoscopic remission and changed the treatment goal to altering the course of the disease and slowing its progression.
In many cases, treatment decisions are made by assessing the disease activity in the first instance.2 However, many other factors are important, such as size and location of mucosal lesions, anatomic distribution and load of inflammation (local or systemic), prior hospitalisations or surgery, postoperative complications, and extraintestinal manifestations and previous treatment response. Other essential considerations include the impact of the disease on the patient’s quality of life and treatment preference.
In the treatment decision making process, all the aforementioned factors are important; however, the physician should consider the future. Currently, there are few, if any, precise biomarkers of progression and the lack of a roadmap of likely disease course confers uncertainty in the treatment direction. Clinical variables (e.g., age,3 disease extent,4-6 C-reactive protein level, faecal calprotectin level, steroid use,3 smoking,7 and mucosal ulceration8,9) may help (Table 1), but these are generalised to the entire IBD cohort. The prognosis of such chronic conditions involves a high degree of unpredictability and uncertainty. In this ‘low validity environment’, simple scores or algorithms can exceed the knowledge of experts,10,11 but there is a clear unmet need for specific predictors of the disease course.
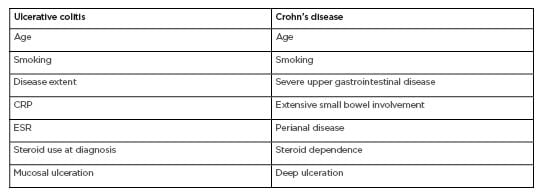
Table 1: Predictors of disease course in ulcerative colitis and Crohn’s disease.3–9, 22–26
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
Panel Discussion: The Secret of Hitting the Mark
Chair: Doctor Peter Irving
Panel: Professor Jonas Halfvarson and Doctor Marieke Pierik
Dr Irving: What do you consider first when making treatment decisions?
Prof Halfvarson: Restricting this to IBD drugs, I would need to initially look at the inflammatory burden. However, I cannot disregard the impact of the disease on the patient overall as I may need to take other actions or add-on treatments for symptoms that are not linked primarily to
the inflammation.
Dr Irving: How do you best assess the inflammatory burden?
Prof Halfvarson: The gold standard is endoscopy for small bowel disease imaging, but few patients can undergo frequent endoscopies to track their disease progression, so often we must rely on proxies, i.e., biomarkers. Which biomarkers are used will vary by region, in Scandinavia there is high use of faecal calprotectin. In other countries, ultrasound is commonly used.
Dr Irving: What are the key factors that drive your choice of treatment?
Dr Pierik: I would always consider disease severity, extent, location, and complications at first when deciding on the strategy for each individual patient. Next, age, psychosocial factors, and comorbidities are considered. In elderly patients, for instance, we also look at the risk of side effects of treatment, and we are reluctant to offer combination therapy, especially for a long period. In very young patients, you also need to consider whether they will take what’s prescribed.
Dr Irving: You talked about elderly patients; what is ‘old’?
Dr Pierik: It’s very difficult to define a cut-off as you need to take into account comorbidities, smoking, and other factors. Every doctor needs to assess ‘old’ on an individual basis, but at around 75 years of age combination therapy becomes very risky.
Dr Irving: What would be a decisive factor for you to decide to follow a top-down approach?
Dr Pierik: I think you decide on a ‘top-down’ approach based on disease severity and risk factors for poor prognosis, but you may select another specific drug based on comorbidities, for example, when presented with an IBD patient with SpA that has failed a classic disease-modifying antirheumatic drug, you will select a biologic in collaboration with the rheumatologist. If a patient has comorbid IBD and autoimmune hepatitis, you might select a thiopurine.
Dr Irving: What does a patient who gets a top-down approach look like?
Dr Pierik: An example would be a young girl who was hospitalised at diagnosis with low weight and extensive small bowel involvement. We started combination treatment with an anti-TNFα and thiopurine immediately after diagnosis. Or you might perform a colonoscopy on a suspected IBD patient and see severe, deep ulceration, which would also necessitate combination treatment immediately.
Dr Irving: In some countries it is difficult to adopt a top-down approach. Are you losing much if you adopt a stepwise approach to treatment?
Dr Pierik: If you start treatment with corticosteroids, but they are not effective after a few days and you quickly step up treatment you will not lose too much. But you must monitor the effect of the treatment very closely.
Dr Irving: How do you determine treatment success? What is a strong indicator of remission?
Prof Halfvarson: It is too simplistic to look at any aspect of disease activity in isolation. There are various dimensions of remission, which include symptomatic relief and cessation of objective signs of inflammation, including endoscopic remission and normalisation of inflammatory biomarkers. The correlation between symptoms and endoscopic activity is poor, so you would need to consider biomarkers when it comes to continuous monitoring of the inflammatory activity over a long period of time.
Dr Pierik: I try to teach young doctors that the treatment goal for IBD is prevention of disease progression, so we need to monitor inflammation, either by endoscopy or biomarkers. However, it is important to remember that we are working with people, so if you treat the inflammation but the patient is still feeling unhappy, then the treatment goal is not met. Quality of life and deep remission or endoscopic remission are two different considerations. You want to control mucosal inflammation while optimising quality of life.
Dr Irving: The flipside to this is that the patient feels well on a certain treatment, but endoscopy or biomarkers suggest they are not well, requiring a treatment change. How do you approach this with the patient?
Prof Halfvarson: I try to explain what we currently know about their expected disease course and the increased risk of surgery or other complications. With information and education, the patient will normally agree that the best option is to alter the treatment strategy.
Dr Irving: What about the ones who still say no?
Prof Halfvarson: The patient’s wishes must be respected.
Dr Pierik: I explain the risks of progression, but if an individual patient really doesn’t want to change, I would suggest another endoscopy or MRI after 6 months and reconsider the options at this point.
Dr Irving: What will contribute further to the development of personalised medicine?
Dr Pierik: Understanding the precise underlying molecular mechanisms of each patient’s disease would be preferred, but this is not feasible now or any time soon. Personalised management is already possible at the clinical level by monitoring and intervening on inflammation and quality of life, but also by considering psychosocial and lifestyle disease influencers, including smoking, exercise, diet, treatment adherence, social support, and psychiatric comorbidities. We do this via remote monitoring with a telemedicine tool. Based on the results, we offer every patient personalised intervention for their disease influencers besides drug treatment. Furthermore, patient education is extremely important. Therefore, we can provide personalised management now, while we await refined treatment selection based on molecular mechanisms.
Dr Irving: What is the more promising direction of future biomarker development: genetic predisposition or microbiota?
Prof Halfvarson: Of those two options, microbiota. If considering potential adverse events of certain drugs, genetic profiling may provide information, but otherwise microbiota.
Hidden Gems of Rheumatology
Doctor Frank Behrens
It is interesting to hear about the challenges faced in IBD, i.e., that it is not one disease and there are challenges in personalising care, as many of these challenges are reflected in rheumatology. This is complicated by the selectivity of different treatments and their expected efficacy when the ultimate diagnosis is uncertain. Rheumatoid arthritis is more a syndrome than a distinct entity and is not well-defined. Psoriatic arthritis, for example, shows great variability in symptoms: patients may have severe synovitis and low joint effusion but enthesitis, and some may have early erosive disease or no structural damage after several years. So, it is difficult to predict the disease course.
We need to acquire early imaging (ultrasound), especially in cases of SpA with the aim of treating early and preventing progression to structural damage of affected joints. One important pathological mechanism of rheumatoid arthritis is an increase in vascularisation at the joints. Due
to the autoimmune nature of the condition, activated T cells enter and cause local inflammation of synovial tissue, eventually leading to arthritis. SpA conditions are considered predominantly autoinflammatory rather than autoimmune diseases because they can be induced by direct mechanical stress, e.g., at the Achilles tendon. Secondary synovitis may occur, but it is often not the key feature during onset. Considering this, we began looking at enthesial sites with high mechanical stress and biomarkers for increased vascularisation via ultrasound. In affected patients, the inflammation and damage scores were much higher than healthy controls.
This is also observed in patients with coeliac disease and IBD. The increased vascularisation, sometimes without arthritic symptoms, suggests that SpA may not strictly be an arthritic disease, and may share biomarkers with an array of inflammatory conditions.12
When considering conditions linked to IBD, i.e., SpA, T cells with the IL-23 receptor are observed at the enthesial sites and might play a critical role in the disease. This triggers inflammation and an increase in IL-17, TNFα, and IL-22, which cause structural damage. Therefore, both mechanical stress and IL-23 receptor-positive T cells are hypothesised to be drivers of these conditions.13
The link between IBD and arthritic conditions is believed to be mediated by dysbiosis of the gut. IL-23 is dysregulated in IBD; therefore, it is hypothesised that increased permeability and barrier dysfunction in the gut lead to circulating IL-23 reaching the IL-23 receptor positive T cells at enthesial sites, activating the immune response and causing inflammation in combination with mechanical stress.12
Therefore, if SpA is present, underlying IBD symptoms need to be investigated and referral should be considered. Major red flags that would trigger referral of a SpA patient from a rheumatologist to a gastroenterologist include chronic diarrhoea, rectal bleeding, perianal fistula, and chronic abdominal pain. Conversely, major red flags for referral of an IBD patient to a rheumatologist include chronic lower back pain, dactylitis, enthesitis, and peripheral joint pain or swelling.14
A study conducted over 20 years showed that almost 30% of patients with IBD developed musculoskeletal inflammation (Figure 1).15 This comorbid condition should be considered when determining treatment; i.e., if a SpA patient has IBD, it may be of benefit to keep them on an anti-TNFα due to the pathological mechanisms involved.
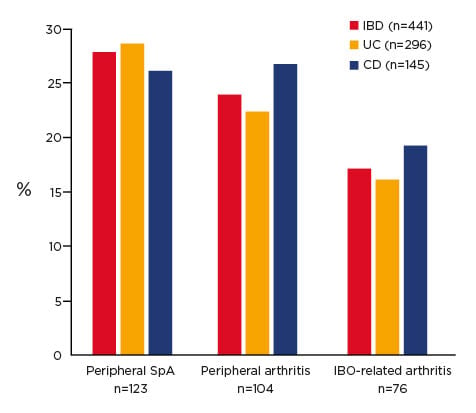
Figure 1. Prevalence of arthritis in inflammatory bowel disease after 20 years of follow up.15
CD: Crohn’s disease; IBD: inflammatory bowel disease; SpA: spondyloarthritis; UC: ulcerative colitis.
In patients with axial SpA, an altered biome versus healthy controls has been observed, similar to those observed in patients with IBD. Therefore, it is important to consider whether the treatment decisions are influencing the microbiome. Preliminary data have shown that more Firmicutes are present when using an IL-17 inhibitor (secukinumab) than an anti-TNFα, which did not alter the biome.16 IL-17 inhibitors are not effective for treating IBD, but are the only biologics after anti-TNFα that are effective in axial SpA, so this knowledge could help to direct treatment in comorbid patients. However, the best treatment pathway could be debated: Should faecal calprotectin be assessed when initiating treatment, and if negative, start IL-17 inhibitor, reserving anti-TNFα as a ‘back-up’ for if the gut becomes involved? Or should treatment initiate on anti-TNFα to reduce the risk of the gut becoming involved?
Personalised Treatment for Inflammatory Bowel Disease: Of Course, but Why and How?
Doctor Claudio Fiocchi
This year’s ECCO can only be described as interesting, especially because many of the topics introduced over the last 3–4 years have become commonplace, such as systems biology, heatmaps, and ‘omics’. The public perception of personalised medicine is concurrently increasing, which must be applied to IBD and other chronic inflammatory diseases.
In an ideal world, there would be one treatment so effective that every patient would benefit. If that is the goal, why is there a focus on
personalised treatment? Every gastroenterologist is aware that diagnosis of IBD is complicated and can take a long time, during which the disease progresses, so a decision to treat as soon as possible is necessary. The use of biologics is common because they are relatively effective at inducing remission; however, treatment with biologics is lopsided because it is only suppressing the immune system, without consideration of the environment, genetics, and microbiome (Figure 2). It is important to consider that by suppressing the immune system, is the disease or just the inflammation being treated?
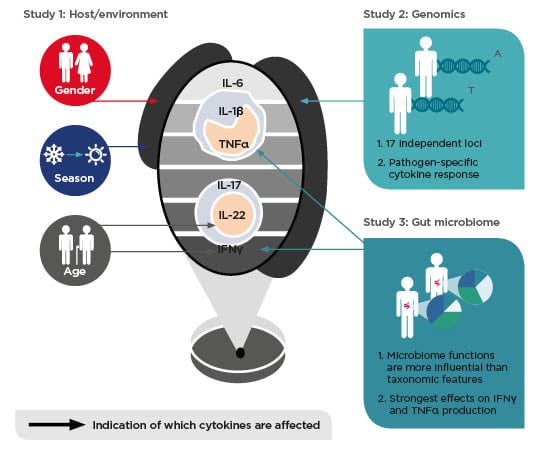
Figure 2: Environmental, genetic, and gut microbial factors impact human cytokine responses
Adapted from Schirmer et al.,19 Ter Horst et al.,27 Li et al.28
Investigation of the molecular components of IBD could help make better diagnoses and treatment decisions. Over 240 genes are associated with IBD,17 but their influence is not yet clear. Furthermore, throughout their lifetime, patients are constantly exposed to unpredictable environmental factors. Whether their impact is positive or negative is unknown. It has been suggested that the most influential factors are those that occur in early life, even in utero. Studies have shown that obese mothers confer intestinal inflammation to their offspring. There is no way at present to know when a patient’s IBD started, only when symptoms appear.
Every subset of the population has a different biome. Environmental, genetic, and microbial factors influence the immune system.18,19 Different geographical locations have different components (urban versus rural) and diets. Measuring microbiome biomarkers is important but very difficult as there is tremendous variation. Two patients may present similarly but do not respond to the same treatment. Natural language processing of medical records shows that diseases diverge considerably over time. As they diverge, the immune system changes, the microbiota changes, and the symptoms change. Hence, indicating a very heterogenous and dynamic condition. Considering a person at one point in time will not provide information on how the disease started and will progress. Clinicians are taught how to diagnose and treat according to what is currently known, but it is becoming more important to think outside this approach.
The term ‘ome’ refers to the totality of any given field, and ‘omics’ to its study, e.g., genomics, proteomics, etc. Systems biology is the computational modelling of big data or complex systems (e.g., chronic diseases like IBD). Precision medicine is an approach to disease treatment and prevention that considers individual variability in genes, environment, and lifestyle. The medical community is generally conservative in terms of adopting novel technologies, but studying the different aspects of IBD separately is not likely to lead to breakthroughs as the information remains compartmentalised.
Using a systems biology approach, integrating information as it is acquired (exposome, genome, microbiome, immunome, etc), and combining it into an ‘IBD interactome’ could lead to new treatment approaches.
Integrating clinical knowledge with transcriptomics, proteomics, microbiomics, etc., is a step in the right direction, but the currently used biomarkers are too generic. Classical medicine relies heavily on one person’s knowledge and experience, which largely leads to similar treatment decisions across groups of patients. Systems biology and deep molecular profiling of the same groups will help to identify patients with the same underlying molecular mechanisms, which will allow for more personalised treatment.20,21
An example that is applicable to current practice, but requires effective cross-collaboration with bioinformaticians, is the construction of molecular networks in IBD. For example, one can identify networks that control cytokines, which can then be targeted by matching key molecules with drug databases. This has led, for example, to the identification of histone deacetylase inhibitors able to target specific components of the disease network.
Treatment must also be considered in the context of changes in the disease over time. For example, a patient may be born without IBD, but is IBD-prone; i.e., has a particular genetic makeup (e.g., an IBD variant) and a particular methylation profile. With exposure to an unknown environmental factor, the patient then displays symptoms of IBD. The methylation profile, which is influenced by the genes and the environment, will change. With that, gene expression changes, the interactome changes and the biological response may now be dominated by TNFα, so treatment with an anti-TNFα will be effective. As time passes, the individual may be exposed to additional triggers of methylation (e.g., contracting a virus or taking up smoking) and the mechanism of IBD alters, requiring an alteration in the treatment provided.
Because IBD is an extremely complex disease, the development of the appropriate tools is vital to provide truly personalised treatment. This is not imminent, but the methodology exists, and it is hoped this will be incorporated into clinical practice within 5–10 years.








