Meeting Summary
The symposium entitled “Dynamic management of CD: reaching a new dimension in patient care” took place on 13th October 2020 during the United European Gastroenterology (UEG) Week Virtual 2020. Distinguished experts Prof Danese, Prof Ghosh, Prof Kucharzik, and Prof Peyrin-Biroulet highlighted the latest developments in Crohn’s disease (CD) management, with a focus on the ground-breaking STARDUST treat-to-target (T2T) trial and the STARDUST intestinal ultrasound (IUS) substudy. The specialists discussed how dynamic management of CD is evolving toward having an emphasis on proactive management, including patient-reported outcomes and endoscopic targets, with the goal of preventing disability and bowel damage. The data from the STARDUST trial illustrated how a T2T strategy could be an additional tool for clinicians, to assist them in making informed treatment-dosing decisions and help patients with CD achieve their treatment goals. The results from the IUS substudy showed how this technique could be useful in the noninvasive monitoring of treatment response in patients with CD. The speakers concluded that, in the current CD treatment landscape, dynamic management strategies can be used to adapt to the patient’s needs, with the goal of providing long-term disease control.
Dynamic Management in Crohn’s Disease
Professor Laurent Peyrin-Biroulet
Prof Peyrin-Biroulet started the symposium by describing how the focus of inflammatory bowel disease (IBD) therapy is moving toward disease modification.1 Early targeting in CD offers a novel opportunity to change the course of long-term disease evolution, helping patients to lead a normal life by preventing disabilities.1 Using paradigms such as T2T, incorporating composite endpoints such as clinical and endoscopic remission, and applying adjunctive measures such as biomarkers, helps provide optimal disease monitoring and management for patients with IBD (Figure 1).2
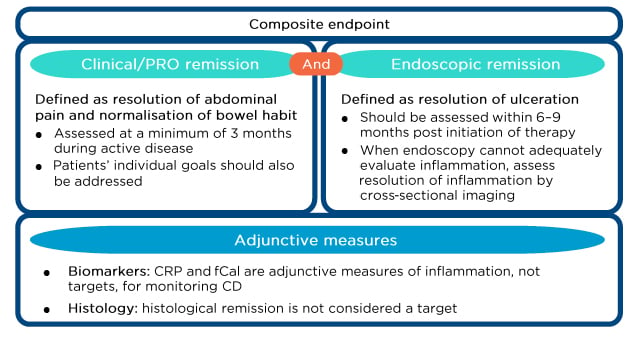
Figure 1: Treat-to-target recommendations in Crohn’s disease.
CD: Crohn’s disease; CRP: C-reactive protein; fCal: faecal calprotectin; PRO: patient-reported outcome.
Adapted from Peyrin-Biroulet et al.2
As evidence has accumulated, the more ambitious goal of complete endoscopic remission has seemed attainable. However, data from the CALM study showed that complete endoscopic remission was achieved in only approximately 20% of patients treated with a tight-control strategy at Week 48, suggesting that this target could have been too ambitious;3 new strategies and therapies with new mechanisms of action may, therefore, be needed to meet endoscopic remission goals. Early disease control may be the best way to change patients’ lives and alter the disease course, as data show that the early use of disease-modifying anti-inflammatory therapies can impact the natural history of CD.1
Novel initiatives in IBD management promote an emphasis on disease modification, firstly to help achieve disease and symptom control, followed by disease remission for long-term disease modification. Defined endpoints can include several measures focussing on patient quality of life and the prevention of disease complications. Recent studies have also shown that transmural healing is a better outcome compared to endoscopic healing as it results in less surgery, fewer hospitalisations, and has a positive impact on the disease course.4
Prior to the development of the STRIDE guidelines, physicians focussed mainly on symptomatic remission and possible achievement of biomarker remission using reactive management strategies. Now, in the era of STRIDE guidelines that recommend tight control and T2T strategies, clinicians are practising proactive management, focussing on achievement of deep remission with an absence of symptoms and severe endoscopic lesions, with the ultimate goal of preventing disability and bowel damage in patients with CD.5
Exploring STARDUST
Professor Silvio Danese
The STARDUST trial6 was the first T2T, randomised trial of adult patients with CD, using endoscopy at Week 16 as a decision point for dose adjustment of ustekinumab, an IL-12/23 inhibitor. The objective of the trial was to test the hypothesis that a maintenance strategy with ustekinumab, based on early endoscopy, regular assessment of biomarkers (e.g., faecal calprotectin [fCal] and C-reactive protein [CRP]) and clinical symptoms (e.g., Crohn’s Disease Activity Index [CDAI]), and subsequent treatment adjustment to achieve the treatment target, is more successful in obtaining endoscopic improvement than a maintenance strategy using standard of care (SoC) with ustekinumab.7
The study also applied IUS examinations as a noninvasive tool to assess the response to treatment in a subgroup of patients.8,9
Patients with moderate-to-severe active CD, who were either biologic-naïve or had been exposed to one previous biologic, were included in the study. Patients received an induction dose (approximately 6 mg/kg intravenously [IV]) of ustekinumab at Week 0, followed by maintenance therapy with ustekinumab (90 mg subcutaneously [SC]) starting at Week 8. An interim analysis was performed at Week 16; if patients achieved the first target (CDAI 70 response), they were randomised to the T2T or SoC arm.
Patients in the T2T arm either received ustekinumab every 8 weeks (q8w) or 12 weeks (q12w), based on the change in the Simple Endoscopic Score for Crohn’s Disease (SES-CD) from baseline. Patients in the T2T arm received maintenance treatment, with possible dose adjustments based on symptoms and biomarkers, while patients in the ustekinumab SoC arm received dose adjustments as per the label, based solely on physician-confirmed disease flare. The primary endpoint at Week 48 was a 50% reduction from the baseline SES-CD value (Figure 2).6
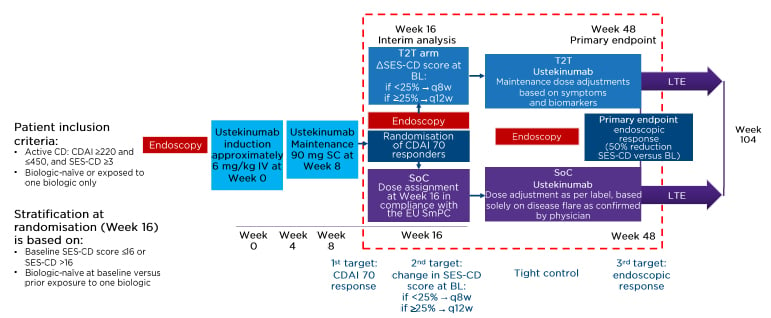
Figure 2: STARDUST study design.
BL: baseline; CD: Crohn’s disease; CDAI: Crohn’s Disease Activity Index; EU: European Union; IV: intravenously; LTE: long-term extension; q8w: every 8 weeks; q12w: every 12 weeks; SC: subcutaneously; SES-CD: Simple Endoscopic Score for Crohn’s Disease; SmPC: summary of product characteristics; SoC: standard of care; T2T: treat-to-target.
Adapted from Janssen-Cilag Ltd.6
During the study maintenance period, CDAI, CRP, and fCal measures were assessed to examine whether patients had achieved the treatment target, defined as a CDAI <220 and ≥70 point improvement in CDAI score from baseline, and a CRP value ≤10 mg/L or an fCal value ≤250 μg/g. If patients had achieved the target, they could continue with the assigned dose. If the target was not reached, they were eligible for dose escalation. If patients did not achieve the target while receiving a dose every 4 weeks, they discontinued the study.10
The patient disposition results showed that 490 patients received ustekinumab induction, with a large proportion of patients achieving CDAI response; 79% of patients in the T2T arm and 87% in the SoC arm completed the study. Discontinuation in the T2T and SoC arms was mainly caused by lack of efficacy and withdrawal by the participant. The dose distribution showed that the majority of patients who received the q12w dose were still receiving that dose at Week 48 (59.8% and 63.8% in the T2T and SoC arms, respectively). Similarly, 40.5% and 78.4% of patients receiving the q8w dose in the T2T and SoC arms, respectively, were still receiving that dose at Week 48.10
The results of the STARDUST study show that the nonresponder imputation (NRI) analysis of the endoscopic response primary endpoint with SES-CD improvement ≥50% at Week 48 was achieved by 37.7% of patients in the T2T arm, compared with 29.9% of patients in the SoC arm (p=0.09). The analyses of the last observation carried forward (LOCF) and the NRI, which only included patients who discontinued because of lack/loss of efficacy, showed that a significant proportion of patients in the T2T arm achieved the primary endpoint, compared with patients in the SoC arm (p=0.049 and p=0.036, respectively).10 There were no between-group differences in the NRI and LOCF analyses for the endoscopic outcomes at Week 48.10 The changes in SES-CD over time from baseline to Week 48 showed a clear and rapid effect of ustekinumab at Week 16 of treatment, characterised by a substantial decrease in mean (95% confidence interval) scores from baseline to Week 16 (13.4 versus 8.8, respectively; Δ-4.6 [-5.5 to -3.7]) in the T2T arm.10 There was no significant difference in SES-CD score between the T2T and SoC arms at Week 48 (8.5 versus 8.6, respectively).
Clinical outcomes at Week 48 for the NRI and LOCF analyses demonstrated that both the T2T and SoC regimens were associated with high efficacy; >70% of patients achieved the CDAI 70 target and >60% of patients were in clinical remission at Week 48 in both groups.10 As seen in the change in endoscopy scores over time, mean CDAI scores also showed a dramatic drop between baseline and Week 8 of treatment in both treatment arms, which was further boosted by treatment optimisation at Week 16 (LOCF analysis).10 Normalisation of biomarker responses (fCal, CRP) at Week 48 was observed in approximately 25% of patients in the NRI and LOCF analyses for both the T2T and SoC arms. Changes in biomarkers over time showed a similar trend, compared with the CDAI 70 data over time for both the T2T and SoC arms, which showed a marked drop between baseline and Week 8 in both treatment groups.10
The safety summary at Week 48 indicated that there were no major differences between the T2T and SoC groups, confirming the well-known safety profile of ustekinumab in patients with CD.10 Prof Danese concluded by reiterating that in the STARDUST study, 48 weeks of treatment with ustekinumab resulted in a numerically higher endoscopic response in patients in the T2T arm compared with SoC, with high clinical remission and biomarker responses also observed in both arms. The safety and tolerability of ustekinumab was consistent with the known safety profile. T2T could, therefore, represent an additional tool for clinicians, to guide ustekinumab dosing decisions for patients with CD.
Can We Reach the Stars with Ultrasound?
Professor Torsten Kucharzik
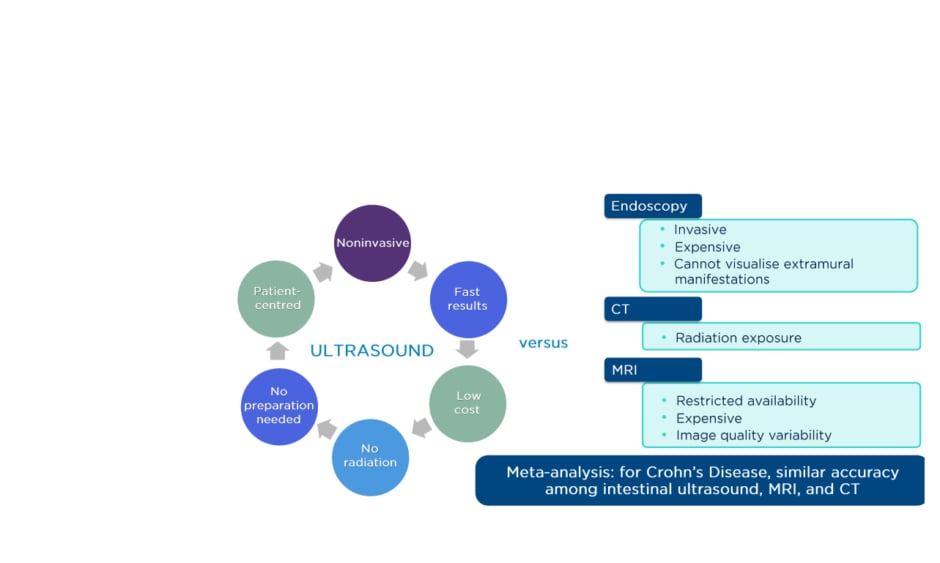
Prof Kucharzik presented the data from the STARDUST IUS substudy, noting that IUS can be used to determine disease activity and severity in patients with IBD, and to detect complications in patients with CD. It is a noninvasive, low-cost, patient-centred technique that has comparable accuracy to MRI and CT scanning (Figure 3).11-13
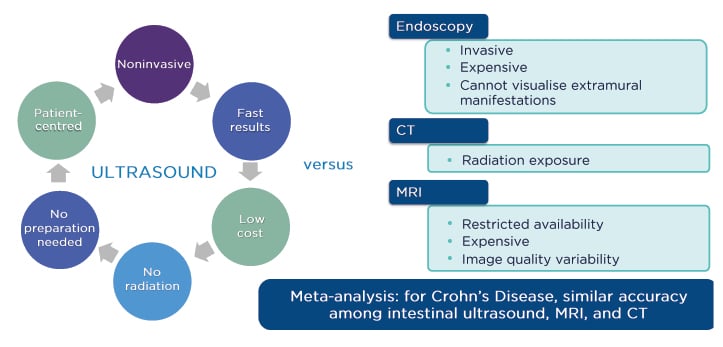
Figure 3: What is currently known about intestinal ultrasound.
Adapted from Panés et al.,11 Horsthuis et al.,12 and Yoon et al.13
The aims of the STARDUST IUS substudy were to assess the effectiveness of ustekinumab in achieving IUS response and remission, and to explore the relationship between IUS response and changes in clinical and endoscopic parameters over time.8,9
IUS can reflect transmural disease activity, and the most prominent parameter to measure this activity is bowel-wall thickness (BWT). The IUS substudy examined BWT in the ileum and the colon in patients with CD; increased BWT was defined as increases of >2 mm in the terminal ileum and >3 mm in the colon, as determined in the transversal and longitudinal sections of the most affected part of the segment. An IUS response was defined as ≥25% reduction in BWT, compared with baseline.14-16 Vascularisation using colour Doppler signal, echo stratification assessment, and inflammatory mesenteric fat assessments were also used to assess transmural disease activity.15
IUS remission, or transmural healing, was defined as normalisation of BWT, normal vascularisation (colour Doppler signal of 0 or 1), normal bowel-wall echo stratification, and the absence of inflammatory mesenteric fat, based on the most affected part of the bowel.17 IUS was performed at baseline, as well as at Weeks 4, 8, 16, and 48.
The baseline characteristics showed that the ileum was the most affected part of the bowel in 50 of the 77 patients participating the substudy (65%), and the colon, including the caecum, was the most affected part in 27 patients (35%).16 The overall percentage change in BWT over time showed a significant decrease compared with baseline values, starting at Week 4 of treatment (p<0.01) and continuing at Week 8 (p<0.0001), Week 16 (p<0.0001), and Week 48 (p<0.0001). There was a more pronounced decrease in BWT in the colon compared with the ileum, but both areas showed significant decreases over time.16
Analyses of IUS response and transmural healing over time showed that 25% of patients showed a response to treatment as early as Week 4, with approximately 24% of patients showing complete transmural healing and complete normalisation of all IUS parameters at Week 48 of treatment.16 The IUS response was observed early, at Week 4, in both the colon and ileum, though the response was more pronounced in the colon.16 There was a progressive increase in the number of patients showing improvements in BWT in both the ileum and colon over time. For the most affected part of the bowel, the proportion of patients with normalisation of vascularisation, bowel-wall stratification, and mesenteric fat increased through to Week 48.16
Prof Kucharzik presented an example IUS image from a selected patient case from the STARDUST study, demonstrating transmural healing in the terminal ileum at Week 48 of ustekinumab treatment, with complete normalisation of all pathological parameters compared with baseline.
The IUS substudy results showed that there was a 92.3% agreement between IUS and endoscopy in defining the most affected part of the bowel at baseline for the ileum, indicating that IUS may be a useful tool, in addition to endoscopy, to help determine the most affected part of the bowel.16 There was also reasonable reliability between IUS response of the most affected part of the bowel at Weeks 4, 8, 16, and 48 and the endoscopic response and biomarker outcomes at Week 48.16
Prof Kucharzik concluded by emphasising that IUS responses to ustekinumab were detected as early as Week 4 of treatment and improved over time up to Week 48. Furthermore, a clinically meaningful percentage of patients achieved transmural healing at Week 48, primarily in the colon.
There was high agreement at baseline between IUS and endoscopy in defining the most affected part of the bowel, as well as reliability between IUS response as early as Week 8 and endoscopic response and biomarker outcomes at Week 48. The results show that IUS can be a useful tool in predicting later endoscopic response with ustekinumab in patients with CD and underline the potential benefits of using IUS as part of a personalised approach to treatment.
Dynamic Management: A Case-Based Approach
Professor Subrata Ghosh
Prof Ghosh demonstrated how the evolving principles of CD management can be applied to current practices, using a case-based approach. He presented the case of a 28-year-old male patient, diagnosed with CD characterised by ileocolonic disease. The patient had started treatment with adalimumab 4 months earlier but had not responded to treatment. Laboratory tests showed modestly elevated CRP and fCal levels, with extensive colonic ulceration during colonoscopy (SES-CD=15), deep ulcers on MRI, and increased BWT and narrowing of the ileum on IUS.
The patient began receiving a single dose of ustekinumab 6 mg/kg IV, followed by maintenance therapy of ustekinumab 90 mg SC q8w after 8 weeks. At 14 weeks of treatment, the patient reported feeling better, with no abdominal tenderness and improvements in laboratory test measures. Colonoscopy revealed ulcerations in the distal ileum, though there was significant healing in the transverse colon.
Prof Ghosh presented several possible treatment choices for the patient, including options for optimising ustekinumab therapy or switching to a different drug class. The patient continued to receive ustekinumab 90 mg SC q8w. At Week 42 of treatment, he reported further reduced symptoms, with corresponding improvements in biomarker responses and BWT, and no ulcerations found on colonoscopy. Prof Kucharzik noted that the initial video showed markedly increased BWT, which had improved at Week 42, indicating a good IUS response, and recommended continuing treatment with ustekinumab q8w to achieve complete transmural healing. The panel agreed that continuation of treatment with ustekinumab q8w, based on the current outcomes, could help the patient achieve this transmural healing goal.
Prof Ghosh highlighted that the initial rapid clinical and endoscopic response to treatment during the induction phase benefited from the IV infusion, and that long-term control and reduction of the disease burden, or even normalisation, can be achieved over time by administering the correct maintenance dosing of the drug at the correct intervals, as well as monitoring appropriately with biomarkers, endoscopy, or ultrasound scan.
Question and Answer Session
The question and answer session included several queries from the virtual audience and focussed on T2T strategies. When asked about the STARDUST results, Prof Danese remarked that one of the strengths of the trial was that it provided robust data regarding the efficacy of ustekinumab in patients who were naïve to biologic therapies and those who had previously received one biologic treatment; there were no significant differences in treatment responses between these patients, with comparable endoscopic and biomarker outcomes. He also commented that the long-term data from the STARDUST trial will be available in the future. When asked about the future of IUS in clinical practice, Prof Ghosh remarked that endoscopy will still have a role in the future; however, going forward, a combination of symptom assessment, patient-reported outcomes, biomarkers, and IUS outcomes is likely to provide the majority of data.
The audience also asked about the future of T2T strategies, commenting that tight monitoring strategies, such as T2T, might represent an increased monitoring burden for patients. Prof Ghosh responded that if patients receive an initial explanation of the goals of the T2T strategy and are included in the treatment decision-making process, most understand the treatment strategy and are generally satisfied with their experience. He added that using IUS techniques also helps them to visualise their improvements. Prof Danese agreed that using IUS provides a noninvasive tool for detailed point-of-care assessments, supporting patients in achieving their treatment goals.