Meeting Summary
Considering patient-reported outcomes (PRO) for optimal disease management is pivotal in many chronic diseases, and inflammatory bowel disease (IBD) is no exception. Validated PRO that assess disease activity and reproducibly reveal how a patient functions and feels are not currently available for patients with ulcerative colitis (UC) or Crohn’s disease (CD). This symposium explored how symptom-based PRO adapted from available scores and tools are evolving for effective and simple implementation in clinical practice. These instruments aim to support physicians in assessing treatment options and selection, and in the provision of long-term, meaningful benefits to patients.
Why Patient-Reported Outcomes Matter in Inflammatory Bowel Disease
Doctor Peter Higgins
Patient-Reported Outcomes Evaluate the Impact of Disease on Patients’ Quality of Life Beyond Clinical Remission
Different outcome assessments, including endoscopic scores, markers of inflammation, and patient-reported symptoms, help physicians to determine the extent, severity, and impact of IBD on an individual patient. IBD symptoms are distressing for patients and burdensome on daily life, so it is important that all aspects of patient health and wellbeing are considered when assessing the individual impact of the disease. Some symptoms of IBD are tangible and measurable, for example, weight loss and fever, and others become obvious during physical examination, such as anal fistulas or abscesses. Most symptoms of IBD, however, can only be reported by patients themselves. Moreover, these symptoms can be difficult to quantify, including pain, diarrhoea, urgency of bowel movements, and fatigue.1
In daily clinical practice, physicians often informally enquire about the developments or changes in such symptoms. PRO measurement instruments provide structure and standardisation to these everyday assessments in the clinic. They aim to make treatment outcomes and/or disease measurements reproducible and comparable across visits and patients. Standardised, validated questionnaires evaluate the patient beyond outcomes like clinical response or remission to measure outcomes that are meaningful to patients.
The Mayo Score and Crohn’s Disease Activity Index are Limited in Capturing the Impact of Disease from the Patient’s Perspective
For patients with IBD, available composite measurements of disease activity include the Mayo Score and Crohn’s Disease Activity Index (CDAI). These tools evaluate symptoms, clinical signs, laboratory findings, and endoscopic assessments. However, they do not capture the impact of the disease from the patient’s perspective and are difficult to apply in routine clinical practice because they are complex, time-consuming, and open to bias.2
For example, the Mayo Score measures disease activity in patients with UC but does not assess how the disease impacts health-related quality of life (HRQoL).2 In addition, scales for the recording of endoscopy results and rectal bleeding (RB), which are important tools in the management of patients with UC, are poorly defined. Both are recorded on a 0–3 scale, where 0 and 3 are clearly defined, but a poor definition and limited dynamic range make a score of 1 or 2 problematic, leading to bias in reporting.3,4 For the measurement of stool frequency (SF), a lack of definition of ‘normal’ frequency makes the scale from ‘normal’ to ‘1−6 stools more than normal’ per day subjective and open to bias.
The CDAI quantifies disease activity for patients with CD.5 It includes objective measures and assessments of HRQoL but, importantly, it does not correlate with measures of bowel inflammation.6 There are also methodological flaws, including low reproducibility and arbitrary cut-off points for remission and response.7 Furthermore, due to the wide scope of the CDAI, the score may be elevated when non-CD factors are present, such as infection or irritable bowel syndrome.7 Despite these limitations and regulatory authorities discouraging its use,7 CDAI has been a useful tool for identifying new effective therapies for CD patients.8 It will continue to be a useful tool until a more accurate assessment of the effectiveness of new medications becomes available.
Health Authorities Recommend Combining Validated Patient-Reported Outcomes Instruments with Objective Inflammation Measures
Although the Mayo Score and CDAI have been widely used as endpoints in clinical trials to develop IBD therapies, health authorities have acknowledged their limitations for everyday clinical practice.2,7,9,10 Such endpoints should not be used alone in clinical trials and should be combined with the patient’s perspective to reflect real life.7,9
Dual measurement of disease activity using PRO instruments and objective measures of inflammation aims to ensure that improvements in disease symptoms (measured and observed) are accompanied by improvements in underlying inflammation, and vice versa.10 In UC, these measurements usually correlate with each other. Generally speaking, PRO measures reflect how patients feel and function at the present time, whereas objective measures of inflammation predict future disease activity and clinical outcomes. The approach of combining both PRO and objective measures is critical to ensuring that all aspects of disease burden, both physical and emotional, are managed effectively and HRQoL improves as a result of therapy.
There are currently no validated PRO instruments for UC and CD and the development of validated PRO measures is a lengthy and complex process. However, Dr Higgins is a member of a consortium collaborating with patient focus groups to develop a novel five-module PRO instrument for IBD. The items, scales, and modules have been revised and refined through a series of testing and feedback. Qualitative and quantitative data have been collected and reported and are in the process of being qualified by the U.S. Food and Drug Administration (FDA) (prequalification expected in 2018). The five modules include bowel signs and symptoms, systemic symptoms, emotional impact, coping behaviours, and impact of IBD on daily life (Figure 1).
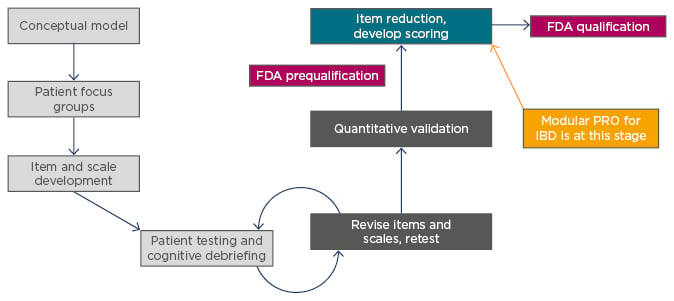
Figure 1: Process of development of a patient-reported outcomes instrument development.
FDA: U.S. Food and Drug Administration; IBD: inflammatory bowel disease; PRO: patient reported outcome.
The basis of this new PRO instrument was provided by the outcomes of the patient focus groups, which were also involved in item and scale development, and testing and retesting of the instrument. After quantitative validation and FDA prequalification, the instrument will be accessible for anyone to use. Final FDA qualification is needed before the instrument can be used in clinical trials.
Symptom-Based Patient-Reported Outcomes Instruments are Useful Tools in the Interim
Symptom-based PRO measures adapted from the Mayo Score or CDAI are considered useful until validated PRO instruments become available. In patients with UC, a two component PRO (PRO-2) combining RB and SF Mayo subscores is currently in use. PRO-2 was not developed as per FDA guidance and, therefore, is not a suitable evidential clinical trial endpoint. Nevertheless, a post-hoc analysis of a mesalazine trial in patients with UC found that the proportion of patients achieving clinical remission as defined by the study protocol had good correlation with the proportion of patients in remission as defined by PRO-2 in combination with Mayo endoscopic subscore.11
In CD, symptom-based PRO measures are also being used. In this scenario, PRO-2 is based on the two symptoms that are most important to patients: abdominal pain (AP) and SF.2 In a retrospective analysis of a methotrexate trial in patients with mild-to-moderate CD, this approach was also shown to be appropriate; clinical remission rates using PRO-2 were similar to those based on a CDAI score ≤150 (the original trial endpoint).12
The use of symptom-based PRO measures is an important step in moving towards a more complete combination of objective and PRO endpoints. Future clinical trials in IBD will have to combine objective markers of inflammation, such as biomarkers or endoscopy results, with validated PRO instruments to encompass how the given drug improves how the patients feel and function.
Optimising Biological Therapies with Patient-Reported Outcomes in Mind
Doctor Brian Feagan
Patients are the Best Source of Information About the Impact of Their Disease
An online survey showed that physicians often underestimate disease severity and overestimate treatment effects compared with assessments completed by UC patients themselves (Figure 2).13 This underlines the authorities’ view that PRO data must originate from the patients to be considered valid.
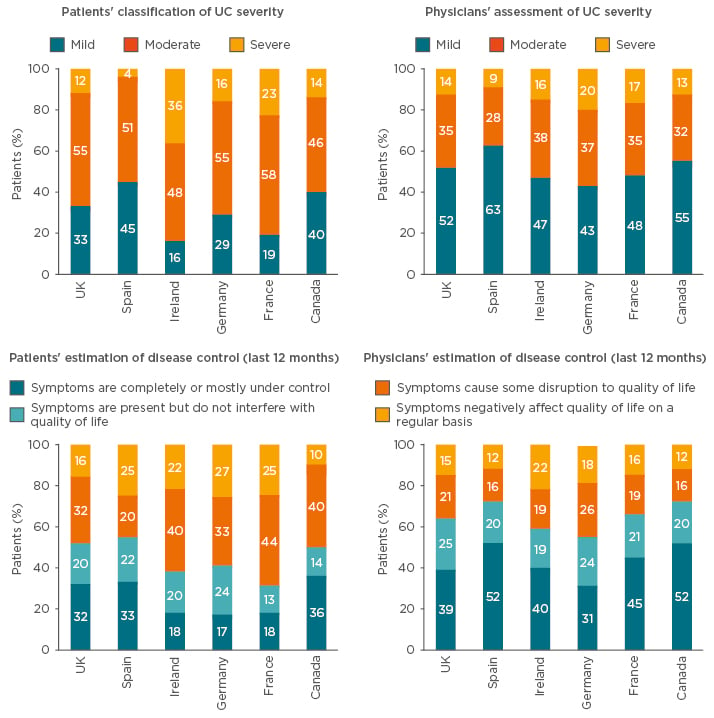
Figure 2: Online survey of patients’ and physicians’ perceptions of disease severity and treatment effect.
An online survey study of 775 adult patients with ulcerative colitis and 475 physicians showed that physicians systematically underestimate disease severity and overestimate treatment effect when compared with the patients’ assessments.
UC: ulcerative colitis.
Adapted from Schreiber et al.13
The FDA now requires PRO instruments to include only patient-derived data. As a result, the Mayo Score and CDAI, which both collect views from patients and physicians, are no longer suitable as evidential endpoints in clinical trials.10 Other instruments commonly used in clinical trials that require input from healthcare professionals, such as the IBD questionnaire (IBDQ), can also no longer be classified as PRO instruments. Clinical trial programmes are adapting to these changes by moving towards symptom-based PRO measures, but, for treatments already used in the clinic, previously acquired study data can only be re-evaluated against the new PRO-based definitions.
Ulcerative Colitis: The Correlation Between Patient-Reported Outcomes and Endoscopy is Reassuring
Historically, an RB subscore of 0 or 1 was included in the definition of clinical remission for the evaluation of a variety of IBD treatments, such as anti-tumour necrosis factor (TNF) therapies in patients with UC. However, with the development of newer biological therapies, the clinical remission threshold has become more stringent and now requires a RB subscore of 0.
Previously acquired clinical trial data have been reanalysed to evaluate the new PRO-based definition of clinical remission in patients with UC. A post-hoc analysis of ULTRA-1 and ULTRA-2 trial data evaluated the relationship of RB and SF subscores with mucosal healing per endoscopy subscore in patients receiving adalimumab with moderate-to-severe UC. A RB subscore of 0 was frequently predictive of mucosal healing; however, SF was a less accurate indicator.14
A more recent post-hoc analysis of data from the GEMINI 1 trial evaluated a RB subscore of 0.15 Within 2 weeks of starting vedolizumab therapy, 30.8% of anti-TNF-naïve patients with moderate-to-severe UC achieved complete resolution of RB (versus 18.4% with placebo). This continued to increase between Week 2 and 6, and the same trend was seen in the overall population, including patients who had previously received anti-TNF therapy.15 This pattern was also mirrored when a composite endpoint of RB subscore of 0 and SF subscore ≤1 was used.
A RB subscore of 0 also conveyed prognostic information. Of the overall patient population with a RB subscore of 0 at Week 14, 56.7% had sustained remission at subsequent visits up to 1 year (versus 20.9% receiving placebo).16 This effect was even more pronounced in the anti-TNF-naïve subpopulation.
Clinical trials evaluating investigational agents are also beginning to use these more stringent PRO measures. For example, the HICKORY trial17 of etrolizumab in patients with UC refractory to or intolerant of anti-TNF therapies used the new FDA definition of PRO instruments. Data have shown that treatment with etrolizumab improved patient-reported symptoms (RB and SF) as early as Week 4, with clinically meaningful improvements in disease activity through to Week 14. Similar to the reanalysis of ULTRA-1 and 2 data,14 improvements in RB subscores were more prominent than for SF subscores.17
Crohn’s Disease: Convincing Study Results for Two-Component Patient-Reported Outcome Endpoints Using a Certain Cut-Off
In early trials of anti-TNF agents in patients with CD, for example the infliximab Targan et al. study18 and the adalimumab CLASSIC study,19 clinical remission was based on CDAI score. However, similar to UC, there has been a shift towards re-evaluating data using symptom-based PRO measures.
A recent post-hoc analysis of data from GEMINI 2, a study of vedolizumab in patients with moderately-to-severely active CD, looked at changes in AP subscore and the number of liquid or SF subscore over the 6-week induction period.20 Similar to the UC data described above, significant improvements compared to placebo were seen as early as 2 weeks after commencing therapy. In the anti-TNF-naïve population, a 20.2% decrease from baseline in the AP subscore was reported with vedolizumab (versus 0.8% with placebo); the subscore continued to decrease to 33.7% at Week 6 (versus 12.6% with placebo). A similar, but not as prominent, decrease in AP subscore was seen in the overall population.20
This pattern for both populations was also observed for SF subscore, and PRO-2 (combining AP and SF subscores) showed a slightly greater effect.20 Analysis of data to Week 52 showed that the symptom-based PRO-2 remission endpoints substantially correlated with the original trial-defined remission endpoint (CDAI ≤150) when a cut-off of AP ≤1 and SF ≤3 was used.21
Analyses of data from recent clinical trials of ustekinumab have confirmed that PRO-based definitions of remission deliver similar results to the original definition of CDAI <150, when PRO cut-offs of AP ≤1 and SF ≤3 were used for patients with moderate-to-severe CD who were refractory to anti-TNF treatment.22 These cut-offs are being used in the recent and ongoing BERGAMOT trials of etrolizumab in anti-TNF-naïve and refractory patients. In the dose-finding Phase II study, there was a clear dose–response relationship for the PRO-2 definition of remission over 14 weeks of induction therapy.23 Thus, for both UC and CD, the interim PRO-2 measures derived from the Mayo Score and the CDAI could be useful endpoints for clinical trials until new PRO instruments are defined and validated.
Continuing Development of Patient-Reported Outcomes in Inflammatory Bowel Disease
Doctor Peter Irving
Recognition of Patient-Centric Healthcare in Routine Clinical Practice
In routine clinical practice, most clinicians informally discuss PRO with their patients. They may ask questions such as ‘How many bowel movements have you been having per day?’ or ‘Has there been any blood in your stool?’. Although these questions are important for assessing bowel activity and are a good example of using PRO in routine clinical practice, they still do not indicate the impact of these symptoms on patients’ quality of life.
Future PRO instruments should support clinicians in daily practice by standardising measurements and focussing patient consultations to gauge the impact of fluctuations in underlying disease on the symptoms that matter most to them. In addition to bowel activity, PRO instruments and measures can evaluate all aspects of patient health, including systemic symptoms, the emotional impact of disease, and coping behaviours. This, in turn, helps physicians to isolate the disease elements that patients consider to have the most impact on their daily lives.
Quality of Life is an Important Measurement of Patient-Reported Outcomes
Whereas physicians have measures at hand to evaluate inflammation in clinical practice (e.g., biomarkers or endoscopy), there are currently no PRO instruments for easy, routine use. Looking at the type of instruments used in clinical trials, there are some questionnaires assessing different aspects of quality of life that could be used in daily practice to evaluate and assess the symptoms that most concern patients. Several questionnaires and instruments have been developed to capture the impact of long-term impairment on HRQoL. For IBD, these include generic scales, such as the Short Form 36 Health Survey questionnaire and the EuroQol five-dimension scale, as well as IBD-specific scales such as the IBDQ. IBDQ32 is a HRQoL instrument with 32 items encompassing bowel symptoms, systemic symptoms, and social and emotional aspects. It has been widely used in clinical trials but has limitations, including complexity and length, as well as an associated financial and administrative burden.2
Change in Inflammatory Bowel Disease Questionnaire Total Score Correlates with Clinical Response but is Rarely Used in Clinical Practice
Evidence from clinical trials has shown that biological agents have a positive effect on HRQoL. In patients with CD, treatment with anti-TNF agents (adalimumab and infliximab) resulted in improvement in patients’ perceptions of their disease state that was sustained with maintenance therapy, as measured by the IBDQ.24,25 Changes in IBDQ score also had clear correlation with clinical responses to treatment in the infliximab ACT 1 and ACT 2 trials in UC.26 Moreover, the GEMINI long-term safety study in patients with UC27,28 and CD29,30 showed that long-term clinical remission with continued vedolizumab treatment correlated with long-term improvements in HRQoL, regardless of prior TNF-antagonist exposure.27-30
Although a change in IBDQ score largely reflects patients’ perceptions of their disease and matches therapeutic response, its limitations hinder its use in everyday clinical practice.2 All questions are weighted equally, so patients’ perceived importance of symptoms, such as abdominal pain and increased bowel movements,2 is not reflected in the scoring.
Post-hoc analyses of the GEMINI 1 and 2 trials demonstrated that individual subcomponents of IBDQ had different levels of improvement in response to vedolizumab therapy. For patients with UC, improvements were reported in all subdomains as early as Week 6 and up to Week 52;31 the greatest improvements were observed in the work or school and fatigue domains, which are particularly important to patients.31 For patients with CD, improvements were also seen as early as Week 6, although fewer domains had sustained improvements to Week 52 than was seen for patients with UC; the greatest improvements in this cohort were observed in the sleep and fatigue domains, both of which are also important to patients.31 It could therefore be more beneficial to study treatment effects on individual selected items of the IBDQ rather than the total score.
Inflammatory Bowel Disease-Control Questionnaire Might be Suitable for Daily Practice
The limitations of the IBDQ2 mean there is an unmet need for a validated instrument to measure quality of life. The International Consortium for Health Outcomes Measurement (ICHOM) has developed a minimum standard set of patient-centred outcome measures for IBD. This IBD-Control Questionnaire uses a range of outcomes, including survival, disease control, and healthcare utilisation.32,33 This tool is simple to use, freely available, quick to complete, and has been validated against the UK IBDQ, the EuroQol five-dimension scale, disease activity scores, and the Physician’s Global Assessment (a rating of disease severity).32,33
Patient-Reported Outcomes e-Monitoring Tools May Also be Beneficial
Advances in technology are providing new possibilities for measuring PRO in clinical practice. The use of technology, such as web portals and smartphone applications, can allow data to be rapidly and efficiently accumulated. This has inspired the Center for Inflammatory Bowel Disease, University of California Los Angeles (UCLA), Los Angeles, California, USA, to develop an IBD scoring system to monitor disease activity.34 Initial results show promise because healthcare resource utilisation outcomes were significantly improved in patients using the emonitoring tools compared with matched controls (Table 1).35
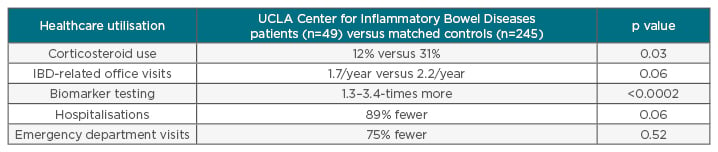
Table 1: Healthcare utilisation in inflammatory bowel disease patients using a patient-reported outcomes e-monitoring tool.
The UCLA Center for Inflammatory Bowel Diseases developed an IBD monitoring index for patient use with mobile health technologies. A comparison with matched controls revealed that UCLA Center for Inflammatory Bowel Diseases patients using this tool had lower healthcare resource utilisation than patients not using the tool.
IBD: inflammatory bowel disease; UCLA: University of California, Los Angeles, California, USA.
Adapted from van Deen et al.35
Conclusion
PRO measures in IBD are increasingly important for evaluating new drugs, as well as guiding treatment decisions in daily clinical practice to improve not only clinical measures of disease activity but also how patients feel and function. Until fully validated PRO instruments are available that reflect and complement objective measures of inflammation, symptom-based PRO measures derived from the Mayo Score and the CDAI are useful tools to assess treatment effect. Continued development of PRO instruments to allow for simple application in daily practice may improve delivery of value-based healthcare and, ultimately, clinical care of patients.








