Meeting Summary
The goal of this symposium was to highlight the importance of early diagnosis, assessing prognostic factors, and treating to target in inflammatory bowel disease (IBD). In the introduction, Prof Colombel outlined the treat to target (T2T) and tight control (TC) approach, which involves predefining treatment targets in consultation with patients, continuously monitoring disease activity, and modifying treatments until targets are achieved. Dr Pariente presented regarding the progressiveness of Crohn’s disease (CD) and described the Lémann index (LI), which assesses cumulative structural damage in CD.1 He outlined the ‘window of opportunity’ in early disease, within which disease progression could be stopped. Dr Pariente said the T2T approach presents the opportunity for a personalised method of treatment; if targets are not achieved, treatment is intensified or switched. Prof Colombel presented the results of the CALM study,2 in which CD patients were randomised 1:1 to clinical management (CM) or TC, meaning treatment was escalated based on clinical symptoms in combination with biomarkers. The primary endpoint of mucosal healing and no deep ulceration was achieved by 45.9% of patients in the TC arm versus 30.3% in the CM arm (p=0.010). Lastly, Prof D’Haens presented a cost-effectiveness analysis using data from CALM. The calculated total direct medical costs for the TC arm were £13,296 versus £12,627 for the CM arm (a direct medical cost difference of £669).3 The quality-adjusted life years (QALY) were 0.684 for the TC arm versus 0.652 for the CM arm (giving a QALY difference of 0.032). The incremental cost-effectiveness ratio showed a cost of £20,913 per QALY gained, which falls within the threshold of The National Institute for Health and Care Excellence (NICE) guidance for cost-effectiveness.
Introduction: Where Are We in 2017?
Professor Jean-Frédéric Colombel
Although progress has been made in the study of IBD, particularly regarding biologics, studies demonstrate treatment gaps and unmet needs. In the past, CD was considered intermittent with flares and remissions; however, gastroenterologists have come to appreciate that it is a progressive disease with the possibility that each flare could result in bowel damage. Studies suggest that damage and disability scores increase from diagnosis (with patients experiencing stricture, fistula or abscess, and surgery) while inflammatory activity (measured by Crohn’s disease activity index [CDAI], Crohn’s disease endoscopic index of severity [CDEIS], and C-reactive protein [CRP]) fluctuates.1
The LI, a score of bowel damage including location, severity, extent, progression, and reversibility, has changed the disease paradigm. The index, developed by the International Program to Develop New Indexes in Crohn’s Disease (IPNIC), demonstrates that each flare leads to bowel damage (strictures, fistulas, abscesses, and surgery).1 Additionally, there are data suggesting ulcerative colitis (UC) is progressive, starting with proctitis, leading to left-sided colitis and extending to pancolitis (the most difficult condition to treat).4
The current goal of CD management is to control progression, leading to the window of opportunity concept whereby if treatment is missed in the subclinical period, the condition will progress. Although IBD management has evolved, the current clinical goal is to obtain sustained remission and reduce steroid use. The new target is endoscopic healing, associated with steroid-free remission, decreased hospitalisations, decreased surgery, and improved quality of life. Future targets are likely to move beyond deep remission (endoscopy findings) to transmural healing, reducing and preventing intestinal damage, which may in turn reduce or prevent disability.5-7
The aim of T2T is to avoid development of serious complications and disability.8 The concept involves treating to predefined treatment targets associated with optimal long-term outcomes (goal- orientated approach), and regular monitoring of the target and/or surrogate marker with optimisation of treatment when targets are not met. Additional principles include tailoring treatment, monitoring individuals, and de-escalating therapy when goals are achieved, if suitable. For success, it is important to involve patients, identify T2T targets together, continuously monitor disease activity, and modify treatment until targets are reached.6-8
In Focus: Importance of Early Diagnosis and Treating to Target in Inflammatory Bowel Disease
Doctor Benjamin Pariente
To understand the importance of applying T2T strategies, there is first a need to consider why IBD patients should be treated as early as possible. While CD and UC are known to be chronic progressive diseases, current indexes only assess inflammatory activity (whether clinical, endoscopic, or biological). Such indexes often have the same values for different patients with early or longer term CD, indicating the need for indexes and tools to capture progression and damage.
Recognition of such requirements led to the development of two new indexes: the first disability index for IBD, and the first digestive damage index in CD, named the LI,1,9-12 which assesses cumulative structural damage in CD. In the LI, the gastrointestinal tract is divided into four regions, each of which is subdivided into serial segments. For each segment, strictures, penetrating lesions, and previous surgery can be scored on a scale with four levels (from null to severe) according to severity, providing an overall index score that can be used to assess damage progression, evaluate treatment, and provide an endpoint for trials.
A study assessing digestive tract damage in 138 CD patients (median age: 34 years) showed that damage was related to disease duration; the LI was 6.3 for patients with a disease duration <2 years, 14.3 for patients with a disease duration of 2–10 years, and 19.0 for patients with a disease duration >10 years.11 The finding of low LI in the first 2 years is important because it demonstrates the window of opportunity just after diagnosis, within which intensive treatment might stop disease progression. The new goal for IBD is to block disease progression and damage. Moreover, a post hoc analysis of the CHARM study13 suggested anti-tumour necrosis factor (TNF) treatments are more efficient early in the IBD course, with results showing differences between placebo and adalimumab for clinical remission rates (at Week 56, results were 19% versus 43% for <2 years; p=0.024; 13% versus 30% for 2–<5 years; p=0.028; and 8% versus 28% for >5 years; p<0.001).
A recent South Korean retrospective analysis classified 670 CD patients into those starting anti-TNF or immune modulators within 2 years of diagnosis and those starting later. The study found that for the early therapy group, times to intestinal surgery (p<0.001), stricturing complications (p=0.002), penetrating complications (p<0.001), and behavioural progression (p<0.001) were significantly longer.14 A French prospective evaluation of 130 CD patients presented at the 25th United European Gastroenterology (UEG) Week showed that damage increased with disease duration (p<0.001) and, furthermore, the LI was 3.7 for patients who received early anti-TNF therapy (<2 years) versus 12.3 for late anti-TNF therapy (p=0.015).15 Such data strongly suggest that early introduction of anti-TNF could prevent damage progression and change the natural history of CD.
The CURE study16 is currently underway and is evaluating the impact of early use of adalimumab on sustained deep remission and long-term outcomes. The aim is to assess if early initiation decreases bowel damage, disability, and surgery over 5 years and whether early use might allow physicians to decrease or even stop treatment to avoid long-term adverse events and reduce healthcare costs. While there is a need for early intensive treatment in some IBD patients, the strategy should not necessarily be applied for all. At an early stage in both CD and UC, indolent disease must be distinguished from aggressive disease. Those with aggressive disease might benefit from a top-down approach, assuring early intensive therapy, while those with indolent disease might benefit from a step-up method, with the possibility of avoiding intensive therapy, immunosuppression, and adverse events.
According to the Paris definition, early CD can be defined as disease duration ≤18 months after diagnosis in patients who have received no previous or current use of immunomodulators and/or biologics with previous or current use of 5-aminosalicylate and/or corticosteroids permitted.17 If this definition alone is used, there is a high risk of overtreatment, highlighting the need to perform prospective cohort studies identifying early predictors of aggressive IBD. A study of newly diagnosed paediatric CD patients showed upregulation of ileal genes controlling extracellular matrix production at diagnosis was associated with stricturing (hazard ratio [HR]: 1.70; 95% confidence interval [CI]: 1.12–2.57; p=0.0120).18 Furthermore, data from a sub-cohort for whom ileal gene expression data were available found that biological markers (i.e., anti-Saccharomyces cerevisiae antibody [ASCA] immunoglobin A and anti-flagellin [CBir1]-positive) were associated with stricturing and penetrating behaviour.18
While awaiting the results of disease modifying trials, we need to adopt new therapeutic strategies, including a T2T approach in IBD, where targets are defined (whether a clinical, biomarker, or composite target) and if not achieved, treatment is intensified or switched.5,8 For CD, T2T recommendations are composite endpoints consisting of clinical or patient-related outcome remission (defined as resolution of abdominal pain and normalisation of bowel habits), assessed at a minimum of 3 months during active disease, and endoscopic remission (defined as resolution of ulceration), assessed within 6–9 months after the start of therapy.19 For UC, the T2T target is clinical or patient-related outcome remission (defined as resolution of rectal bleeding and normalisation of bowel habit), assessed at a minimum of 3 months during active disease, and endoscopic remission (defined as resolution of friability and ulceration at flexible sigmoidoscopy or colonoscopy), which should be assessed within 3–6 months after the start of therapy.19
The ongoing REACT2 study20 in CD compares an enhanced T2T treatment with conventional step care to determine whether T2T can reduce CD-related complications, including hospitalisations, at 1 year. Importantly, the T2T strategy is a personalised approach, with desired outcomes in early disease being complete absence of symptoms, no disease progression, no complications or disability, and normal quality of life; the desired outcome in late-disease is stabilisation of non-inflammatory symptoms, no progression or damage or disability, and improved quality of life.18,21,22
News Update: CALM Study Results
Professor Jean-Frédéric Colombel
In CD it is unknown whether TC, in which treatment decisions are based on close monitoring of inflammatory biomarkers, results in improved patient outcomes. CALM23 was a prospective, open-label, multicentre, active-controlled Phase III study evaluating two treatment algorithms in CD, CM, and TC. Patients were randomised 1:1 to CM (where escalation was driven by the CDAI and prednisone use; n=122) or TC (escalation was driven by monitoring of CDAI, faecal calprotectin [FC], CRP, and prednisolone use; n=122).
For the TC arm, even if patients were doing well, treatment was escalated if one of the biomarkers was raised. Treatment in both arms was escalated in a stepwise manner from no treatment to adalimumab induction, followed by adalimumab every other week, adalimumab every week, and lastly to both weekly adalimumab and daily azathioprine. During the post-randomisation treatment period, treatment was escalated at 12, 24, and 36 weeks if patients met any of the post-randomisation treatment failure criteria.
For the CM arm, CALM failure was considered to occur at Week 1 if CDAI decreased by <70 points compared with baseline or CDAI was >200. For the TC arm, treatment failure was considered to occur at Week 1 for CDAI ≥150, CRP ≥5 mg/L, FC ≥250 µg/L, and prednisone use at Week 0. For Weeks 11, 23, and 35, CALM failure criteria for CM were a CDAI decrease <100 points compared with baseline or CDAI ≥200 and prednisone use a week prior to the visit, while for TC failure, the criteria were CDAI ≥150, CRP ≥5 mg/L, FC ≥250 µg/L, and prednisone use a week prior to the visit.23
Inclusion criteria included adults aged 18–75 years with ileal, colonic (including rectal), or ileocolonic CD with moderate-to-severe CD with or without systemic corticosteroids. Exclusion criteria included previous or current use of biologics or immunomodulators, more than two previous courses of corticosteroids, or current use of corticosteroids for >3 months before screening.23 The primary endpoint was mucosal healing (CDEIS <4) and no deep ulcerations (assessed 48 weeks after randomisation). Secondary endpoints included deep remission, biologic remission, mucosal healing, complete endoscopic response, and steroid-free remission, among others.
For CALM, of 460 patients screened, 244 met the inclusion criteria and were randomised to CM (n=122) or TC (n=122). The study was completed by 93 CM patients (76.2%) and 90 TC patients (73.8%), with reasons for discontinuation including adverse events, withdrawal of consent, loss to follow-up, and lack of efficacy. Baseline characteristics showed mean disease duration was 0.86 years in the CM group and 1.04 years in the TC group, making CALM subjects one of the earliest populations ever studied in anti-TNF clinical trials. CALM results showed that the primary endpoint of mucosal healing (CDEIS <4) and no deep ulceration was achieved in 45.9% in the TC arm versus 30.3% in the CM arm (p=0.010) (Figure 1).
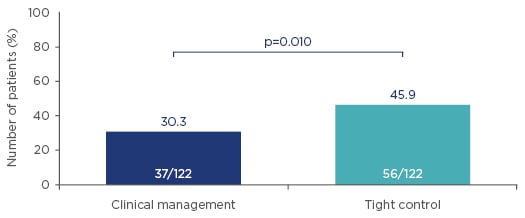
Figure 1: CALM primary endpoints at 48 weeks after randomisation (mucosal healing [CDEIS <4] and no deep ulcerations). Higher rates of mucosal healing and no deep ulceration were observed in early Crohn’s disease when treating to a target of biomarker levels (C-reactive protein and faecal calprotectin), compared with symptom-driven clinical management.
CDEIS: Crohn’s disease endoscopic index of severity.
Adapted from Colombel et al.24
The TC arm was also strongly positive for a number of secondary endpoints at Week 48, including deep remission (p=0.0104), biologic remission (p=0.006), mucosal healing (p=0.010), and endoscopic response (p=0.067). However, CALM results were not significant for mucosal healing in all segments (p=0.229) and complete endoscopic remission (p=0.728) (Figure 2). Furthermore, at all time points, significantly more patients achieved steroid-free remission in the TC than CM arm (p=0.009 at 11 weeks, p=0.003 at 23 weeks, p=0.007 at 35 weeks, and p<0.001 at 48 weeks).
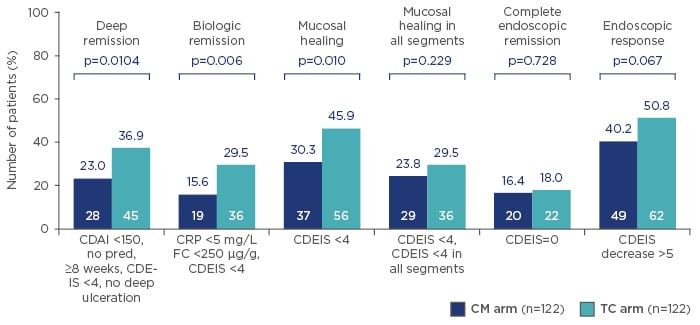
Figure 2: CALM secondary endpoints at 48 weeks after randomisation. Higher rates of mucosal healing and deep remission were observed in early Crohn’s disease when treating to a target of biomarker levels (C-reactive protein and faecal calprotectin), compared with symptom-driven clinical management.
CDAI: Crohn’s disease activity index; CDEIS: Crohn’s disease endoscopic index of severity; CM: clinical management; CRP: C-reactive protein; FC: faecal calprotectin; pred: prednisone; TC: tight control.
Adapted from Colombel et al.24
An analysis exploring different treatment regimens at Weeks 0, 1, 24, and 36 showed treatment was escalated and tapered earlier in the TC arm compared with the CM arm. Since more patients in the TC group escalated to adalimumab every week at 12 weeks and to adalimumab every week plus azathioprine at 24 weeks than in the CM group, investigators predicted side effects would be higher in the TC group. This, however, was not the case. Serious adverse events occurred in 20.5% of the CM group versus 18% of the TC group and most notably serious infections occurred in 9.8% of the CM group versus 4.9% of the TC group.
To conclude, CALM represents the first study demonstrating that the TC approach, using objective data (inflammation biomarkers), improves outcomes in CD compared with symptom-driven care. Results give rise to the view that managing CD patients by clinical symptoms alone may not adequately control underlying inflammation and that biomarker levels can guide treatment escalations, leading to superior endoscopic and clinical outcomes. Finally, the TC approach did not lead to increased safety signals.
Treating to Target in Inflammatory Bowel Disease: At What Cost?
Professor Geert D’Haens
Several patients had escalated treatment to weekly dosing in CALM, raising the question of whether such expenditure is cost-effective. Financial costs represent only one aspect of IBD, with the need to also take quality of life, physical wellbeing, and psychological impact into consideration. In IBD, benefits from optimal use of therapies through T2T include obtaining sustained deep remission, reductions in accumulation of intestinal damage, complications and disability, reduced need for surgery, relief of extraintestinal manifestations, therapy withdrawal, improved quality of life, and improved work and social function. In children and adolescents, an additional goal is restoring growth, and for all patients, the ultimate goal is a return to normal life.
The CALM study demonstrated that symptomatic and endoscopic remission was reached more frequently using TC than CM. Furthermore, the study showed TC resulted in earlier and increased use of adalimumab. More intensive immunosuppression with adalimumab, however, did not result in increased safety signals during the year of the trial. Looking at serious infections, anal abscesses occurred in four patients of the CM group (3.3%) versus zero of the TC group, and most other infections occurred equally frequently between the groups.
CALM showed:
- Significantly fewer CD-related hospitalisations occurred in the TC group (13.2 events per 100 patient years) than the CM group (28.0 events per 100 patient years) (p=0.021).23
- When pooling adverse outcomes, at 48 weeks fewer hospitalisations or complications were observed in the TC group (14.8%) than the CM group (20.5%), although the difference was not statistically significant (p=0.240).23
- The rates of CD-related surgical procedures after randomisation were 6.6 events per 100 patient years for the TC arm versus 8.7 events per 100 patient years in the CM arm (p=0.582).23
- The TC and CM time to CD-related hospitalisation or serious complications began to separate at Week 15 after remission, favouring TC, but the difference was not statistically significant (HR: 0.7; 95% CI: 0.4–1.3; p=0.249).23
However, there was an early significant separation in the time to CD flare between the CM and TC groups (HR: 0.4; 95% CI: 0.2–0.8; p=0.012), with flare defined as an increase in CDAI score by ≥70 points from 1 week prior to randomisation and a total CDAI score of >220.23
The 1-year follow-up used in CALM was shorter than the REACT trial,25 in which patients were assigned to early combined immunosuppression with a TNF antagonist and antimetabolite or conventional management, and followed for 2 years. Patients were reviewed every 3 months and if they were not in clinical remission, treatment was escalated. No difference in any adverse outcomes was seen at 12 months, but results at 24 months favoured rapid treatment escalation based on symptoms. For the REACT2 study, which is anticipated to be reported in 2018, treatment escalation is based on endoscopy.20
For the CALM economic analysis, patients were categorised by disease state according to CDAI, with remission (defined as CDAI <150), moderate disease (CDAI ≥150 to <300), severe disease (CDAI ≥300 to <450), and very severe (CDAI ≥450). The model used a previously published health state structure,26 and leveraged an analysis relating CDAI to the EuroQOL five dimensions questionnaire (EQ-5D) on health-related quality of life,27 and a study relating CDAI to resource utilisation28 to estimate QALY and other direct medical costs. CD-related hospitalisations and adalimumab injections were based on data from the CALM trial.
At the simplest level, when people do not feel well, they visit the doctor, with hospitalisation representing one of the main drivers of healthcare utilisation and expense. Calculations for the incremental cost-effectiveness ratio (ICER) take into account effects and costs of interventions but also improvements in quality of life. Gains in QALY can be calculated from CDAI distribution (from the CALM study) multiplied by EuroQOL five dimensions questionnaire (EQ-5D) scores according to CDAI.27
Direct medical costs are the sum of hospitalisation costs, adalimumab costs, and other direct medical costs. The ICER is calculated based on the difference between the TC and CM arms by dividing the direct medical costs by QALY. Results showed predicted time in remission was 62.1% for the TC arm versus 47.3% for the CM arm (difference: 14.8%); the CD-related hospitalisations were 0.13 for the TC arm versus 0.28 per person year for the CM arm (difference: -0.15); the number of adalimumab doses (40 mg) were 30.87 for the TC arm versus 24.72 for the CM arm (difference: 6.15); and other direct medical costs were £1,298 for the TC arm versus £1,524 for the CM arm (difference: -£226), CD-related hospitalisation costs were £1,128 for the TC arm versus £2,398 for the CM arm (difference: -£1,270), and adalimumab costs were £10,870 for TC arm versus £8,705 for the CM arm (difference: £2,165).3
Over the 48 weeks, the total direct medical costs were £13,296 for the TC arm versus £12,627 for the CM arm, giving a difference of £669. QALY were 0.684 for the TC arm versus 0.652 for the CM arm, giving a QALY difference of 0.032. The difference in costs of £669 divided by the difference in QALY of 0.032 produced an ICER of £20,913 per QALY (Figure 3). According to UK NICE guidelines, interventions costing less than £30,000 per QALY gained can be considered cost-effective.30 In conclusion, in the CALM study TC in CD led to improved clinical outcomes, reduced CD-related hospitalisation, and improved patient quality of life. Furthermore, based on CALM data, a TC strategy appears cost-effective compared with CM.
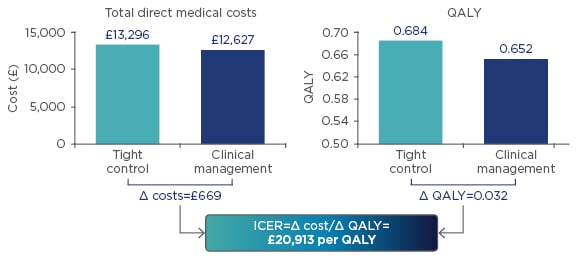
Figure 3: Economic analysis from the CALM study.
ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life years.
Adapted from Panaccione et al.29
Discussion
Considering how the CALM study could be translated into clinical practice, Prof Colombel said that the most important message was the need to regularly monitor symptoms and biomarkers.








