Meeting Summary
The Janssen-sponsored symposium entitled “New dimensions in UC: exploring recent data and measures in IBD” took place during the 15th Congress of the European Crohn’s and Colitis Organisation (ECCO) in Vienna, Austria, on 13th February 2020. Distinguished experts Prof Vermeire, Prof Armuzzi, and Prof Allez highlighted the need for improved disease control in patients with ulcerative colitis (UC), and emphasised that treatment goals may soon include histological improvement, in addition to prolonged clinical and steroid-free remission and mucosal healing. Prof Armuzzi noted that treatment goals are shifting from a focus on symptom control to measures encompassing both endoscopic and histological healing as potential targets for disease modification. Prof Allez emphasised that several histological and endoscopic measures are available that can aid in predicting inflammatory bowel disease (IBD) outcomes, and discussed how maintenance treatment with the IL-12/23 inhibitor ustekinumab was associated with higher rates of endoscopic, histological, and histo-endoscopic mucosal healing in patients with UC, compared with placebo treatment. Prof Vermeire demonstrated how recent developments in UC management can be applied in clinical practice, using a case study to illustrate important practical points; this included an examination of when to perform biopsies and the use of histological readouts to help direct discussions about treatment plans, including the continuation, de-escalation, and cessation of treatment.
Ulcerative Colitis: New Drugs, New Targets, and Better Outcomes?
Professor Alessandro Armuzzi
UC is a chronic, progressive disorder with an unknown aetiology.1 Few population-based cohort studies have assessed both disease course and the use of immunomodulators. However, one study examining 717 patients with cumulative exposure to medical treatment and progression to complications during a 5-year follow-up period showed that, though conditions were treated more aggressively with immunomodulators or biological therapies, disease outcomes and colectomy rates did not show significant changes.1 Furthermore, hospitalisation and surgery rates did not decrease, indicating that UC may not have been under control after 5 years of treatment in these patients.1 Current treatment paradigms include the use of aminosalicylates for mild disease, which can escalate to treatment with corticosteroids or aminosalicylates combined with immunomodulators, and treatment with biologics or small-molecule therapies as the disease worsens.2-4 Patients with very severe disease may need to undergo surgery.4
The current therapeutic goals in UC include the rapid remission of clinical symptoms and endoscopic remission with induction therapy, prolonged clinical and endoscopic steroid-free remission, prevention of complications, optimal surgical timing, and improved patient quality of life with maintenance therapy. Recent research has put forward evidence of endoscopic healing as an appropriate treatment target that drives the course of UC.5 This research showed that endoscopic healing was associated with improved long-term outcomes, including long-term clinical remission (odds ratio [OR]: 4.50; 95% confidence interval [CI]: 2.12–9.52), colectomy-free rate at follow-up (OR: 4.15; 95% CI: 2.53–6.81), and long-term mucosal healing (OR: 8.40; 95% CI: 3.13–22.53).5
A recent meta-analysis study of 2,132 patients with UC revealed that only 36% of patients showed endoscopic remission with complete abscence of symptoms, reflecting the heterogeneity of the disease.6 Although in 2015 histological healing was not recommended as a treatment target due to insufficient evidence, accumulating evidence has shown that histological healing is associated with endoscopic healing and can help predict long-term outcomes.7 However, endoscopic and histological remission do not completely correlate in UC outcomes; studies have shown that histological activity may be present despite clinical and endoscopic remission.8,9 The evolving UC management landscape has shown that histological remission, though not included in the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) recommendations, is predictive of reduced corticosteroid use and is associated with reduced risk of relapse in patients achieving endoscopic improvement and remission (Figure 1).10-22 The future UC treatment recommendations may, therefore, soon include histological remission as an important target for optimal management.23
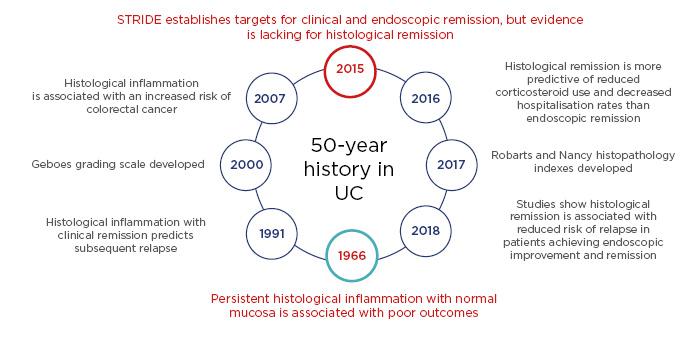
Figure 1: The history of histology in ulcerative colitis.10-22
STRIDE: Selecting Therapeutic Targets in Inflammatory Bowel Disease; UC: ulcerative colitis.
Genetic evidence links IL-23 to inflammatory disease risk.24 Several treatments including guselkumab, brazikumab, risankizumab, and mirikizumab (all of which target the IL-23 p19 subunit), and ustekinumab (which targets the IL-23 p40 subunit) all target T-cell differentiation, resulting in a profound effect on inflammation.24,25 For example, induction treatment with the IL-12/23 inhibitor ustekinumab, given as either a 130 mg or ~6 mg/kg intravenous (IV) dose, resulted in histo-endoscopic healing at Week 8 of treatment in patients with UC in the UNIFI study.26
The results of the Phase III UNIFI study, which included a novel histo-endoscopic healing outcome,27 also showed that maintenance treatment with ustekinumab 90 mg/kg given subcutaneously (SC) every 12 or 8 weeks resulted in endoscopic and histological improvement at Week 44 of treatment, compared with placebo;28 patients receiving ustekinumab SC during the UNIFI trial had higher rates of endoscopic improvement, histological improvement, and histo-endoscopic mucosal healing at Week 44, compared with patients receiving placebo.28 Furthermore, symptomatic remission was sustained through Week 92 among randomised patients who continued to receive ustekinumab during the UNIFI long-term extension trial.29 A 1-year treatment regimen with ustekinumab, including the ~6 mg/kg induction dose, was associated with a higher probability of clinical response and remission, in a network meta-analysis comparison with all advanced treatments, in patients with moderate-to-severe UC who had previously failed on one biologic therapy.30
In conclusion, Prof Armuzzi noted that there are still several unknowns in the treatment and management of UC, and that there are a number of remaining unmet needs in current treatment strategies. However, the evolution of therapeutic targets has now created a shift from focussing on symptom control to more objective measures of disease control, including a definition of ‘complete remission’ that encompasses both endoscopic and histological healing as potential targets for disease modification. Furthermore, treatment with the IL-12/23 inhibitor ustekinumab showed efficacy in induction and maintenance of histo-endoscopic mucosal healing and long-term steroid-free symptomatic remission in patients with moderate-to-severe UC.
How Good Have We Become in Predicting Long-term Outcomes in Ulcerative Colitis?
Professor Matthieu Allez
Predicting disease outcomes in patients with UC can be complicated due to individual heterogeneity, with different phenotypes and different levels of disease severity and extent, including individual responses to treatment as well as several genetic, environmental, cellular, and molecular factors.31-33 Data from the Inflammatory Bowel Disease in South-Eastern Norway (IBSEN) cohort, which included 423 patients with UC and spanned a decade, showed that approximately 55% of patients achieved remission or had mild disease after initial high activity, while 37% of patients experienced a chronic intermittent disease course.34 Poorly controlled UC can result in long-term consequences, including shortening of the colon and narrowing of the rectum, altered colonic activity, and anorectal dysfunction.35 Disease activity and extent, disease course, experience with previous medications, extra-intestinal manifestations, and patient experiences should, therefore, all be considered in the UC treatment plan.
In Crohn’s disease (CD), deep and extensive ulcerations during colonoscopy are indicators of an aggressive disease course; patients with deep and extensive ulcers have a significantly greater risk of colectomy and penetrating complications over time, compared with patients without lesions.36 Deep extensive ulcerations in patients with severe endoscopic colitis and swollen mucosa in patients with moderate endoscopic colitis are also predictive factors for the outcome of intensive IV attacks of UC.37,38 In acute severe UC, the UC endoscopic index of severity (UCEIS) at admission and faecal calprotectin (fCal) levels on Day 3 are predictive of steroid response.39 For example, one study showed that fCal levels >1,000 μg/g on Day 3 and UCEIS levels ≥6 on admission were associated with IV corticosteroid failure and the need for medical rescue therapy or colectomy.39
Histological inflammation has been associated with an increased risk for colorectal neoplasia.40 Histological activity, including deep ulceration, frequent crypt abscesses, and wide disease extent is also predictive of clinical outcomes in patients with UC.9,41 Studies have also shown that histological normalisation is associated with relapse-free survival,20 and that histological remission is a better predictor of steroid-use and hospitalisation than endoscopic remission.12
In the Phase III UNIFI trial with the IL-12/23 inhibitor ustekinumab, induction therapy with 6 mg/kg IV and maintenance therapy with 90 mg/kg SC given every 12 or 8 weeks, resulted in significantly higher rates of clinical remission at Weeks 8 and 44, respectively, compared with placebo.27 A substantial proportion of patients with UC who were in clinical remission at maintenance baseline showed improvement at Week 44 of ustekinumab treatment, compared with placebo.42 Furthermore, patients who were in clinical remission at maintenance baseline had better clinical outcomes than those patients who were not in clinical remission at the maintenance intiation timepoint.42
The results of the UNIFI study also showed that histological improvement is associated with lower disease activity and greater clinical improvement at induction Week 8. Histological improvement after induction therapy was associated with positive outcomes, including endoscopic improvement, histo-endoscopic mucosal healing, and (steroid-free) clinical remission at Week 44 of treatment (Figure 2).27 The results of the UNIFI study also showed that histological improvement is associated with lower disease activity and greater clinical improvement at induction Week 8, and that patients who acheived histo-endoscopic healing at Week 8 were more likely to have improved outcomes at Week 44.27 Ustekinumab treatment also promoted normalisation of colonic genes to a greater extent compared with placebo, and in patients who had achieved clinical remission at Week 44, compared with those who had not yet achieved remission.28
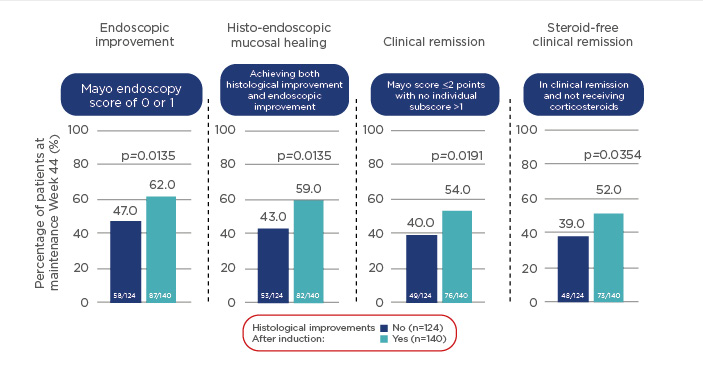
Figure 2: Histological improvement in patients with ulcerative colitis after induction therapy with ustekinumab is associated with positive outcomes at Week 44; p-values based on t-test.
Randomised patients to ustekinumab 90 mg subcutaneously every 12 or 8 weeks in the maintenance study.27
In conclusion, Prof Allez emphasised that there are several histological and endoscopic measures available in the clinic that can aid in predicting IBD outcomes. In the UNIFI study, the 90 mg SC maintenance dose of ustekinumab was associated with higher rates of endoscopic and histological healing, as well as histo-endoscopic mucosal healing in patients with UC, compared with placebo. Patients with histo-endoscopic mucosal healing after induction were also more likely to achieve more positive subsequent clinical outcomes with ustekinumab maintenance therapy than those with only endoscopic improvement at Week 44. This outcome further underscores that histo-endoscopic mucosal healing represents a broad, new clinical endpoint that is predictive of subsequent clinical outcomes.
Is All This Applicable to Daily Clinical Practice?
Professor Séverine Vermeire
The ever-evolving principles of IBD management, including new targets, therapeutic options, and outcome measures, all represent exciting tools that can be used to help physicians optimise management of their patients. To help illustrate how the information given in the previous presentations could be applied in daily clinical practice, Prof Vermeire used the case study of a 39-year-old female patient with UC proctitis, who had been receiving oral and topical 5-aminosalycilic acid (5-ASA) and started treatment with golimumab a few years later. Two years after golimumab treatment initiation, the patient had achieved a Mayo score of 0 and showed mucosal healing on colonoscopy; however, a little more than 1 year later the patient presented with psoriasiform eczema on her face and scalp, at which point golimumab treatment was discontinued. After 3 years of only the occasional need for 5-ASA suppositories, the patient began to experience red anal blood loss and several bowel movements per day; these symptoms were not alleviated with the use of suppositories. Prof Vermeire then asked the attendees what further information might be necessary for them to make a treatment decision for the patient. More than 70% of attendees indicated that they would perform an endoscopy as well as a biopsy prior to making the next treatment decision.
Baseline assessments for optimal management include patient-reported outcomes (PRO), such as rectal bleeding and stool frequency,43,44 and inflammatory biomarker levels, such as fCal10,45 and C-reactive protein levels.44,10 Patients should receive endoscopic and histological assessments, which could be assessed via the Mayo Clinic endoscopic subscore and UECIS score, and histological assessments such as the Geboes grading, Robarts histopathology index, and Nancy score. Biopsies should be performed in those who are refractory, and/or those who have severe disease.13-15,46,47 In this patient case, a biopsy was not mandatory according to the current guidelines.
Returning to the case, the PRO included red anal blood loss, 4–6 bowel movements per day, and a CRP level of <5 mg/L. Following discussion of treatment options with the patient, she started on ustekinumab therapy; the choice was made based on multiple factors including disease severity and extent, the patient’s preferences and expectations, and the medication formulation and route of administration. Treatment choices should ultimately reflect these factors, focussing on the improvement of clinical, PRO, and endoscopic targets.7,10
Four weeks after initiating ustekinumab treatment, the patient showed improvements in blood loss and bowel movement frequency; after 8 weeks of treatment she reported only 2–3 bowel movements a day, with no urgency or blood loss and marked endoscopic improvements. Since the treatment goals of maintenance therapy include steroid-free remission (defined both clinically and endoscopically), histological and biomarker measures, as well as biopsies, can be performed to ensure optimal maintenance (Figure 3).7,10,44 For example, histological activity may be present despite the achievement of clinical and endoscopic remission in UC and correlations between the Geboes and Mayo endoscopic subscores have been shown to be poor.9
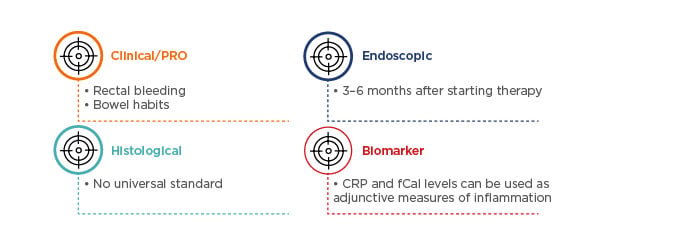
Figure 3: Follow-up assessments for optimal maintenance in ulcerative colitis management, with the goal of clinical and endoscopic steroid-free remission.7,10,44
CRP: C-reactive protein; fCal: faecal calprotectin; PRO: patient-reported outcomes; UC: ulcerative colitis.
Obtaining biopsies from patients may be useful, as histological improvements have previously been linked with improved clinical outcomes,10 and evidence of histological healing may provide long-term reassurance for patients.12 Having a histological readout may also help direct discussions about treatment plans, including the continuation, de-escalation, and cessation of treatment. Prof Vermeire concluded by sharing that, in the case of this female patient, ustekinumab treatment was given every 8 weeks, with sigmoidoscopy and bioscopies planned for later in 2020. The case study patient’s plan is to de-escalate treatment to every 12 weeks when all the treatment targets have been met.








