Meeting Summary
Inflammatory bowel diseases (IBDs) are chronic disabling conditions. Despite the benefits of anti-tumour necrosis factor-α agents in improving quality of life and reducing the need for surgery in many patients, only one-third achieve clinical remission after 1 year of treatment. It is important that treatments go beyond just the alleviation of symptoms, and help to achieve mucosal healing and deep remission.1 The symposium reviewed the natural course of IBD and discussed how focussing management strategies away from simple symptomatic control towards maintaining mucosal healing can significantly improve the quality of life and wider clinical outcomes of patients with IBD. However, this shift in approach requires the redefining of disease severity to highlight the importance of inflammation control and mucosal healing in preventing long-term damage and disability.
Dr Peyrin-Biroulet opened the sessions by reviewing how the Randomised Evaluation of an Algorithm for Crohn’s Treatment (REACT) study has enhanced the understanding of the natural history of IBD, and how complete mucosal healing provides the best outcomes in IBD.2 Dr Colombel highlighted that uncontrolled inflammation in IBD can lead to poor outcomes, and how simple demographic and clinical features can guide the clinician in identifying patients at higher risk for disease complications both at diagnosis and throughout the disease course. Dr Ghosh discussed the importance of defining disease severity in IBD and reinforced that while symptoms related to disease activity are a component of overall disease severity, many factors need to be considered to understand the total impact on a patient’s quality of life.
Changing the Inflammatory Bowel Disease Patient’s Life by Altering the Course of Disease
Doctor Laurent Peyrin-Biroulet
The current management of IBD focusses on symptom management. However, it is important to look beyond symptoms and focus on long-term disability brought on by bowel damage, stricture, fistulae, and abscesses, which lead to hospitalisation and surgery.1 It was stressed that surgery is not a cure, and approximately one-third of patients will experience early (≤30 days postoperative) and later (>30 days postoperative) complications. Later complications occur in 17–55% of patients with ulcerative colitis (UC) undergoing surgery and include pouchitis (29%), faecal incontinence (21%), and small bowel obstruction (17%).3 To reduce such complications, it is important to change the course of the disease, and thereby minimise the need for surgery.
Evidence to support the move towards disease modification comes from the REACT-1 trial in Crohn’s disease (CD), a cluster randomised controlled trial that compared the efficacy of an algorithm using early combined immunosuppression (ECI) with adalimumab plus antimetabolite with conventional management.4 The primary outcome was the proportion of patients in corticosteroid-free remission (Harvey-Bradshaw Index score [HBI] ≤4) at 12 months at the practice level. The 12-month practice-level remission rates were similar at ECI and conventional management centres (66% versus 62%, respectively). Importantly, the 12-month patient-level composite rate of major adverse outcomes, defined as occurrence of surgery or serious disease-related complications, was lower at ECI practices than at conventional management practices (31% and 27%, respectively).4 The hazard ratio (HR) (95% confidence interval [CI]) for the occurrence of surgery was 0.69 (0.50–0.97), p=0.031; whereas the HR (95% CI) of the occurrence of serious disease-related complications was 0.73 (0.61–0.87), p=0.001. The results suggest that time-bound algorithmic care using earlier combination immunosuppression with anti-tumour necrosis factor might be the best way to alter the natural course of these diseases by preventing disability and bowel damage. There is an 18-month window of opportunity for early intervention with disease-modifying anti-IBD drugs in patients with poor prognostic factors.1 The CURE study is investigating the impact of early use of adalimumab on sustained deep remission and long-term outcomes including bowel damage, disability, and surgery.5 New biologic-based therapies should show promise in changing disease course and patients’ lives by improving both quality of life and avoiding disability.
Prevention and Prognostication of Inflammatory Bowel Disease in Clinical Practice
Doctor Jean-Frédéric Colombel
IBD has traditionally been considered an intermittent disease, with flares and remission that can be measured using inflammatory indexes, such as the Crohn’s Disease Activity Index (CDAI), the Crohn’s Disease Endoscopic Index of Severity (CDEIS) or biomarkers such as C-reactive protein levels. While the clinical activity of the disease is remitting and recurring, the damage is progressive, accumulating with each flare-up. In CD, damage is manifested as strictures, fistulae, and abscesses, ultimately requiring hospitalisation and surgery, which has a high rate of disease recurrence.6 Therefore, strategies for the management of IBDs are shifting from simple control of symptoms towards full control of these diseases (i.e. clinical and endoscopic remission), with the final aim of blocking their progression early and preventing bowel damage and disability.6 The challenge of early intervention is that not every patient needs top-down intervention (avoidance of intensive therapy, immunosuppression, adverse events), but that in clinical practice during the first patient visit it is important to determine the degree of risk of complications in each patient in order to develop an appropriate management plan.
Simple demographic and clinical features can guide the clinician in identifying patients at higher risk of disease complications at diagnosis and throughout the disease course. Torres et al.7 conducted a comprehensive review of the literature and identified predictors of long-term IBD prognosis. Summary statements relating to prognosis were produced to guide clinicians on the best use of predictors in individual patients, however many of the risk factors were identified retrospectively and still need validation. It is important to note that the definition of a poor outcome was not consistent in the literature, but, regardless of the definition, there are many predictors of poor outcomes. Patients with CD presenting at a young age or with extensive anatomical involvement, deep ulcerations, ileal/ileocolonic involvement, perianal and/or severe rectal disease, or penetrating/stenosing behaviour should be regarded as having a high risk of complications. Patients with UC presenting at a young age, with extensive colitis and frequent flare-ups needing steroids or hospitalisation, are at increased risk for colectomy or future hospitalisation. Smoking status, concurrent primary sclerosing cholangitis, and concurrent infections may impact the course of disease. Interestingly, the review by Torres et al.7 also determined that current genetic and serological markers lack the required sensitivity and specificity to be currently incorporated into clinical use.
Siegel et al.8 have designed a web-based communication tool to display individualised CD predictive outcomes based on clinical, serologic, and genetic variables to aid the patient in visualising their individual risk of a disabling course of disease. The methodology of the web-based application addresses the inherent dynamic complexity of interactions between variables, and the advantages of traditional statistical methods to provide real-time prediction of outcomes. The overall goal of this web-based model is to transform complex clinical data into patient-friendly information that can help providers and patients make personalised decisions about treatment options.8 The tool is still evolving, but so far the project has recruited 133 patients, both male and female, with a mean age of 31 years, 51% diagnosed within 18 months, with low-risk (19%), medium-risk (56%), and high-risk patients (25%).8 The tool is anticipated to be available at the end of 2016, and there are plans to develop a similar tool for UC.
Predicting outcomes in IBD is important because moderate-to-high-risk patients need intensive therapy before they develop sustained tissue damage. It is possible to use simple, yet mostly not validated, demographic and clinical features to stratify patients and help guide the therapeutic strategy. However, the field is evolving, and new predictive models will help identify the right patients for the right treatment at the right time and support communication to aid patient involvement in decision-making. To further inform personalised therapy, there is a need for composite scores incorporating a wider range of factors and endpoints. In the future, it may be possible to use genetic and serologic markers to predict IBD development even before clinical symptoms appear with prevention of any bowel damage being the aspirational goal.
Maintaining Quality of Life for Inflammatory Bowel Disease Patients by Redefining Disease Severity
Doctor Laurent Peyrin-Biroulet
Patients at a high risk of poor outcomes in IBD need early intervention to prevent disease progression and lasting damage. Most treatment algorithms stratify patients by disease severity, typically based on clinical symptoms (disease activity) at a given time, although no formal validated or consensus definitions of mild, moderate, or severe IBD currently exist for use in routine clinical practice.9 Intestinal damage begins soon after onset of inflammation, sometimes in the absence of significant symptoms, especially in CD. The development of validated and reproducible measures of IBD severity, accounting for both current signs and symptoms and assessment of cumulative bowel damage is essential.
To reach a consensus on the definition of disease severity, a literature review was undertaken to first identify the attributes of disease severity3,10 and second determine the importance of the individual attributes in defining severely active disease.11 The search revealed three main domains relevant to the evaluation of disease severity in IBD: impact of the disease on the patient, disease burden, and disease course (Figure 1). The domains are not mutually exclusive and the correlations and interactions are not proportionate.
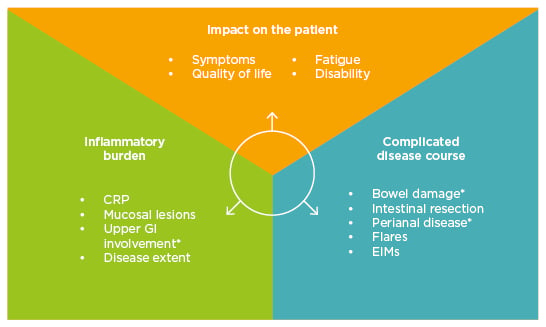
Figure 1: Domains encompassing disease severity in inflammatory bowel disease.
*Only for Crohn’s disease.
CRP: C-reactive protein; EIMs: extra-intestinal manifestations; GI: gastrointestinal.
CD and UC are progressive disorders resulting from cumulative intestinal damage.9 However, disease severity components differ according to disease state. An asymptomatic patient with CD has extensive small bowel disease and moderately active endoscopic lesions. The patient has high inflammatory burden but there is almost no current impact on the patient’s quality of life because they are asymptomatic but it seems that they have, or could have, a complicated disease course. In a further case, the patient has a short ileal stricture associated with occlusive disabling symptoms. The impact on the patient’s quality of life is high even without current inflammation and yet they are still at a high risk of a complicated disease course (Figure 2).
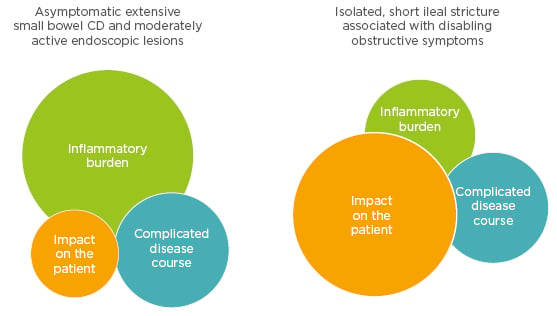
Figure 2: Components of disease severity.
CD: Crohn’s disease.
International definitions of disease severity, such as the American College of Gastroenterology (ACG)12 or the European Crohn’s and Colitis Organisation (ECCO)13 predominantly rely on symptoms. The impact of the disease on patients with CD has been measured by arbitrary thresholds of activity according to clinical symptoms while neglecting to assess bowel damage. While symptoms related to disease activity are a component of overall disease severity, many factors need to be considered to understand the total impact of IBD on patients.
The International Organization for the study of Inflammatory Bowel Disease (IOIBD) convened a meeting in 2014 to seek a consensus and develop an index to define disease severity. The new disease severity index identified the attributes associated with disease severity, calculated the average importance of each attribute, and translated it into a baseline set of disease severity indices for future adaptation for use in clinical practice. The relative importance of each attribute was converted to two 100-point scales, where absence of a symptom equals zero. Based on the panel’s opinion, overall CD severity was associated more with intestinal damage in contrast to overall UC disease severity, which was more dependent on symptoms and impact on daily life. Many factors, including but not limited to disease activity, need to be considered to understand the total impact of IBD on patients. Once validated, disease severity indices may provide a useful tool for consistent assessment of patients with IBD and prevent irreversible bowel damage.11
Interactive Case Discussion
Doctor Subrata Ghosh
Dr Ghosh introduced the audience to IBD Via, a web-based, decision-analysis training tool, with patient case exercises to practice IBD management strategies and outcome prediction. The exercises included are based on real cases supported by clinical evidence and designed and approved by IBD experts. Prognosis cases incorporate IBD Ahead guidance on predicting outcomes and full cases additionally include guidance on optimising conventional therapy and monitoring strategies.
Dr Ghosh also conducted an interactive exercise with the audience by presenting the case of a 32-year-old man, an insurance broker, who smoked 12–15 cigarettes a day, presenting to the family physician with joint pain for 6 months in his elbows and knees. He was taking non-steroidal anti-inflammatory therapies prescribed by the family physician, and was reported to have had a painful red eye 1 year ago that was treated by an ophthalmologist (the primary-care physician when referring this patient sent a photo of the eye). The patient also had right lower quadrant pain and diarrhoea for 8 months, loose bowel movements, and increasing fatigue. The patient had no relevant family history and had an appendectomy at 23 years of age.
The interactive case study illustrated that in planning management strategies in clinical practice, it is important to move away from simple symptomatic control and move towards maintaining mucosal healing and achieving deep remission (no bowel inflammation, no impact on the activities of daily life) and the best strategy may be to alter the natural course of these diseases by preventing disability and bowel damage. While symptoms related to disease activity are a component of overall disease severity, many factors need to be considered to understand the total impact on a patient’s quality of life.
Conclusion
Dr Peyrin-Biroulet concluded the day’s presentations by highlighting that in treating patients, clinicians should assess prognosis to identify patients needing early intervention,7 consider mucosal healing efficacy when selecting therapy,2 and think about long-term outcomes when treating progressive and disabling chronic conditions.9 Treatment with medications with proven effectiveness to heal and maintain the mucosa before irreparable damage takes place must be the primary treatment goal in IBD.








