Presenters: Salvatore Crucillà,1 Fiona Kinnear,2 Tatyana Kugler,3 Peter Macinga,4 Cristina Rubín de Célix,5,6 Ayesha Shah,7,8 Joakim Svahn,9 James White10
1. Gastroenterology Unit, Azienda Ospedaliera Universitaria Integrata Verona, Italy
2. Novozymes A/S, Cork, Ireland
3. Department of Internal Medicine, Donetsk National Medical University, Ukraine
4. Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Prague, Czechia
5. Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS-Princesa), Universidad Autónoma de Madrid (UAM), Madrid, Spain
6. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain
7. Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Brisbane, Australia
8. Faculty of Medicine and Faculty of Health and Behavioural Sciences, University of Queensland, Brisbane, Australia
9. Viatris AB, Stockholm, Sweden
10. Medscape Global Education, London, UK
Disclosure: Crucillà declares no conflicts of interest. Kinnear is an employee of Novozymes, which manufactures the Bifidobacterium longum 1714® and 35624® strains. Kugler declares no conflicts of interest. Macinga declares no conflicts of interest. Rubín de Célix has received educational funding from Ferring, AbbVie, Norgine, Tillotts Pharma, MSD, Pfizer, Takeda, and Janssen. Shah declares no conflicts of interest. Svahn is an employee of Viatris AB. White declares no conflicts of interest.
Acknowledgements: Writing assistance provided by Nicola Humphry, Nottingham, UK.
Support: The publication of this article was funded by Abbott. The views and opinions expressed are exclusively those of the speakers.
Citation: EMJ Gastroenterol. 2022;11[Suppl 9]:4-13. DOI/10.33590/emjgastroenterol/10033131. https://doi.org/10.33590/emjgastroenterol/10033131.
Meeting Summary
Interdisciplinary sessions at the recent United European Gastroenterology (UEG) Week, held in Vienna, Austria, from 8th to 11th October 2022, covered new approaches to the diagnosis and treatment of gastrointestinal and hepatic disorders, with a focus on advances in the non-invasive management of these diseases. Abstract-based and poster sessions presented original research from Europe and other parts of the world. This review summarises selected data presented in the fields of inflammatory bowel disease (IBD), disorders of gut–brain interaction (DGBI), gastro-oesophageal reflux disease (GORD), and disorders of the pancreas. Previously termed functional gastrointestinal disorders, the Rome Foundation is gradually moving toward the use of DGBI in place of functional gastrointestinal disorders to avoid stigma. Cristina Rubín de Célix, Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS-Princesa), Universidad Autónoma de Madrid (UAM), and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Spain, described a systematic review and meta-analysis of real-world evidence for ustekinumab in the treatment of Crohn’s disease. Regarding irritable bowel syndrome (IBS), Salvatore Crucillà, Gastroenterology Unit, Azienda Ospedaliera Universitaria Integrata Verona, Italy, showed that following a Mediterranean diet resulted in symptom improvement and faecal microbiota changes in patients with constipation-predominant IBS. Fiona Kinnear, Novozymes A/S, Cork, Ireland, reported patients’ experiences of probiotics for IBS. Tatyana Kugler, Department of Internal Medicine, Donetsk National Medical University, Ukraine, discussed quality of life (QoL) in patients with functional dyspepsia (FD), and Ayesha Shah, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Brisbane, and Faculty of Medicine and Faculty of Health and Behavioural Sciences, University of Queensland, Brisbane, Australia, considered the impact of a diagnosis of more than one DGBI. James White, Medscape Global Education, London, UK, considered the impact of continuing medical education (CME) on confidence in the management of GORD. Additionally, Peter Macinga, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Prague, Czechia, provided evidence that chronic pancreatitis is associated with an alteration in the gut microbiota, and Joakim Svahn, Viatris AB, Stockholm, Sweden, described the use of a patient support programme (PSP) in patients prescribed pancreatic enzyme replacement therapy (PERT) for pancreatic exocrine insufficiency (PEI).IRRITABLE BOWEL DISEASE
The incidence of IBD is increasing sharply in developing countries and continues to rise in developed countries.1 The pathophysiology of IBD is believed to be multifactorial, involving genetics, the gut microbiome, and environmental factors.2 The available treatments for IBD have grown over the past decade, but it is important to personalise disease management through prognostication of the patient’s disease course and therapeutic response.3
Real-World Evidence of the Effectiveness and Safety of Ustekinumab for the Treatment of Crohn’s Disease: Systematic Review and Meta-Analysis of Observational Studies
Cristina Rubín de Célix
Ustekinumab is a human monoclonal IgF1κ antibody selective for p40, a protein subunit found in both IL-12 and IL-23.4 Ustekinumab binds to free IL-12 and IL-23, preventing these cytokines from interacting with cell surface receptors and driving Th1 and Th17 pathways.4
Based on data from clinical trials, ustekinumab is approved for use in adults with moderately-to-severely active Crohn’s disease, for whom conventional therapy or a TNFα agonist was unsuccessful or is contraindicated.4 However, Rubín de Célix explained that real-world data on the outcomes of ustekinumab therapy in patients with Crohn’s disease is limited, and that further evidence is needed.
Rubín de Célix presented the results of a meta-analysis, which aimed to evaluate the safety and effectiveness of ustekinumab reported by observational studies in patients with Crohn’s disease.5 Rubín de Célix explained that observational studies, excluding those using ustekinumab exclusively as prophylaxis, were identified by searching the PubMed and Embase databases. Of the 63 studies (8,529 patients) included, most assessed ustekinumab in patients with Crohn’s disease treated previously with biologics. Efficacy was assessed from the response rate, remission rate, and endoscopic remission rate in the short term (8–12 weeks), medium term (16–24 weeks), and long term (48–52 weeks [hl]Figure 1[/hl]).
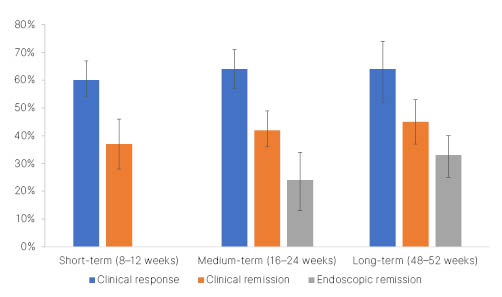
Figure 1: Effectiveness of ustekinumab in patients with Crohn’s disease.
Adapted from Rubin de Célix et al.5
Sub-analyses, stratified by study design, prior treatment with biologics, dose administered, and population, were unable to fully account for the heterogeneity in response rates between studies. Across all the studies, nearly one-third of patients required ustekinumab dose optimisation, which was effective in 60% of cases. On average, 18% of patients experienced a loss of response during follow-up. Predictors of poor clinical response included prior exposure to biologics, stricturing or penetrating disease, and high disease severity at first dose (measured by the Harvey-Bradshaw Index [HBI]). Twenty-five percent of patients experienced adverse events, of which infections were most frequent (4%). The majority of adverse events were mild, and they resulted in treatment withdrawal in 7% of patients.
Rubín de Célix concluded that ustekinumab is an effective and safe therapy in patients with Crohn’s disease in clinical practice. The pivotal clinical trials of ustekinumab (6 mg/kg) in this population, UNITI-1 and UNITI-2, reported clinical response rates of 37.8% and 57.9%, respectively, and clinical remission rates of 20.9% and 40.2%, respectively, at Week 8.6 After 44 weeks of maintenance therapy (90 mg every 8 weeks), the IM-UNITI study reported a clinical response rate of 59.4% and a clinical remission rate of 53.1%.6 Rubín de Célix emphasised that the results of this meta-analysis underline that clinical benefit from ustekinumab seems to be higher in observational studies than in clinical trials.
DISORDERS OF GUT–BRAIN INTERACTION
DGBIs, formally known as functional gastrointestinal disorders, are a spectrum of conditions characterised by an abnormality of gastrointestinal function.7 DGBIs involve visceral hypersensitivity, motility disorder, an abnormal microbiome, or brain–gut dysfunction.8 DGBIs, which include GORD, FD, IBS, and chronic idiopathic constipation,9 affect up to 40% of people at any time, most of whom will have chronic, fluctuating symptoms.10 Psychological comorbidity is common, but whether this is a driver or consequence of DGBI is not clear.10 Current treatment options include lifestyle modifications, microbiota manipulation, prokinetics, laxatives, antispasmodics, and neuromodulators, although therapies targeting the underlying pathological mechanisms are scarce.11
Anxiety, Depression, and Quality of Life in Patients with Functional Dyspepsia: A Cross-Sectional Study
Tatyana Kugler
DGBIs such as FD are often associated with changes in mental health,12,13 yet, as explained by Kugler, the contribution of anxiety and depression to the QoL of patients with DGBIs remains unclear. Kugler pointed out that this represents a significant medical and social problem, because DGBIs more commonly affect younger people of working age.14,15
Kugler presented findings from a cross-sectional survey of 125 patients with FD (defined using Rome IV criteria) and 70 healthy volunteers.16 All participants completed standard questionnaires designed to evaluate anxiety/depression symptoms (Hospital Anxiety and Depression Scale [HADS]), QoL (Short Form-8 [SF-8] questionnaire; standard 4-week form), and severity of epigastric pain or abdominal discomfort (Leuven Postprandial Distress Scale [LPDS]).
Anxiety and depression were significantly (p<0.001) more prevalent in patients with FD (50.4% and 42.4%, respectively) compared with healthy volunteers (13.3% and 6.7%, respectively [hl]Figure 2[/hl]). On average, the levels of anxiety and depression were also significantly higher in the population with FD than in the volunteers (anxiety score: 7.9 and 4.2, respectively; p<0.001; depression score: 6.9 and 3.4, respectively; p<0.001). QoL scores were significantly lower in patients with FD than in healthy volunteers (p<0.001), and both emotional and psychological components contributed to this difference. Linear regression analysis identified a positive correlation between the severity of disease symptoms and the levels of anxiety (R=0.353; p<0.05) and depression (R=0.291; p<0.05). Similarly, a negative correlation was observed between the severity of dyspepsia and the patient’s QoL (R=-0.264; p<0.05). Female patients experienced significantly higher levels of anxiety than male patients (mean HADS score: 8.54 versus 6.54; p<0.05). Anxiety and depression levels were also significantly higher in patients with severe FD symptoms than in those with moderate symptoms (mean HADS score for anxiety: 7.96 versus 7.92; p<0.05; mean HADS score for depression: 7.06 versus 6.94; p<0.05).
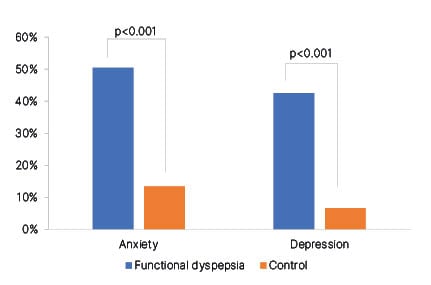
Figure 2: Prevalence of anxiety and depression in patients with functional dyspepsia versus healthy volunteers.
Adapted from Kugler et al.16
Kugler concluded that anxiety was more prevalent than depression in patients with FD, and that self-reported symptom severity, anxiety, and depression were independently associated with QoL. Kugler suggested that measurement of both symptom severity and anxiety/depression levels represent important tools for assessing the severity of FD and the effectiveness of therapy.
A Dual Strain Bifidobacterium longum Probiotic Is Associated with Significant Improvements in Gastrointestinal and Psychosocial Symptoms in People with Irritable Bowel Syndrome: A Real-World Experience Programme
Fiona Kinnear
It has been suggested that the gut microbiota may play a critical role in the dysregulation of the gut–brain axis that contributes to the pathogenesis of IBS.17 Changes in the intestinal microbiota have been associated with some of the gastrointestinal and psychosocial symptoms of IBS,17 and differences in microbiota composition have been identified between a subset of patients with IBS and age- and gender-matched controls.18 These findings indicate that gut microbiota represent a potential target for IBS therapy. Kinnear explained that clinical trials have demonstrated beneficial effects of supplementation with Bifidobacterium longum 35624 alone or in combination with B. longum 1714 in patients with IBS,19-21 but that real-world evidence was lacking.
Kinnear presented data from a real-world experience programme, which aimed to evaluate gastrointestinal and psychosocial symptoms in patients with IBS receiving a daily supplement combining B. longum 35624 and 1714 (1×109 colony forming units) for 12 weeks. Patients were referred to the programme by UK-registered dietitians. Symptoms and impact on daily life over the previous 2 weeks were assessed at baseline and at 4-week intervals, using an online questionnaire.
Of 63 participants (75% female) who completed surveys at baseline and Week 4, eight participants (13%) were prescribed antibiotics during the programme and excluded from the primary analysis. All the remaining participants (n=55) reported experiencing gastrointestinal symptoms, including abdominal distension (85%), abdominal pain (73%), diarrhoea (65%), and constipation (45%). Furthermore, 85% of the participants experienced psychosocial symptoms such as IBS-related stress (67%), fatigue (65%), anxiety/low mood (49%), and sleep disruption (45%). Significant improvements (p≤0.02) were observed for all symptoms after daily supplementation with B. longum 35624 and 1714 for 4 weeks, with mean changes from baseline (on a 10-point Likert scale) of -1.38 for abdominal pain, -1.47 for abdominal distension, -1.56 for bowel habit dissatisfaction, -0.71 for stress, -0.89 for fatigue, ‑0.96 for mood, and -1.85 for IBS impact on daily life.
Kinnear concluded that these real-world data are consistent with those from clinical research, indicating that combined supplementation with B. longum 35624 and 1714 may produce clinical benefits in people with IBS.
Overlap of Functional Gastrointestinal Disorders Reflecting Brain–Gut Interactions: A Systematic Review and Meta-Analysis
Ayesha Shah
DGBIs are defined as distinct combinations of chronic or recurrent gastrointestinal symptoms not explained by structural or biochemical disturbances.22 There are 33 adult and 20 paediatric DGBIs classified according to the Rome IV criteria, including IBS, FD, functional constipation, functional bloating, and functional diarrhoea.23 It has been suggested that the simultaneous occurrence of multiple DGBIs,24 such as FD and IBS, in a given patient may be associated with increased symptom severity and greater psychosocial and work-related impairments.25
Shah presented data from a systematic review and meta-analysis, which aimed to clarify the prevalence of DGBI overlap; the association of DGBI overlap with psychosocial comorbidities; and the impact of diagnostic tools, clinical appraisals, and geographic and socioeconomic factors. PubMed and Embase were searched for studies published up to February 2020 that reported the prevalence of overlapping DGBIs. A random effects model was used to calculate odds ratios for patients with one DGBI and those with more than one DGBI.
A total of 32 studies, reporting data for 47,160 adult patients diagnosed with DGBIs, were identified. Pooled prevalence of DGBI overlap was 36.5%, although there was substantial heterogeneity between studies (I2=99.51; p=0.0001).
QoL was significantly lower in patients with overlapping DGBI diagnoses than in those with a single DGBI, both in the mental (pooled standardised mean difference [SMD]: ‑0.25; p=0.024) and physical (SMD: -0.63; p=0.0001) component scores. The prevalences of anxiety and depression, as measured by the HADS, were higher in patients with overlapping DGBI diagnoses than in those without (SMD: 0.50 and SMD: 0.53, respectively; each p=0.0001). Geographic region, gross domestic product, sex, and age were not associated with the diagnosis of more than one DGBI.
Shah concluded that approximately one-third of patients had more than one DGBI as defined by the Rome criteria, and that these patients had more severe impairment of QoL and a higher rate of anxiety/depression. Shah explained that this indicates that dysfunction of gut–brain interactions may be particularly relevant in patients with overlapping DGBIs. However, Shah stressed that, despite the large sample size, there was considerable heterogeneity between studies, and the results should, therefore, be interpreted with caution.
The Role of Dietary Habits on Symptom Control and Gut Microbiota Composition in Patients with Irritable Bowel Syndrome with Constipation
Salvatore Crucillà
Although the exact aetiology of IBS has not yet been elucidated,23 both diet and gut dysbiosis appear to play a role in the pathophysiology of this condition.17,26,27 Crucillà presented results from a pilot study conducted in Italy that aimed to observe the effects of a Mediterranean diet on gastrointestinal symptoms and changes in gut microbiota in patients with constipation-predominant IBS (IBS-C).
Fifteen patients with IBS-C and 17 healthy subjects (controls) followed a Mediterranean diet for 4 weeks. The diet was guided by a nutritional protocol designed to follow the proportions of macronutrients and quantity of fibre recommended by the Italian Society of Human Nutrition (SINU) in the 2014 revision of the Dietary Reference Values of Nutrients and Energy for Italian population.
Two weeks prior to the intervention and at the end of the study, participants completed a food diary and an IBS symptom questionnaire, and faecal samples were collected. Microbiota analysis was performed on faecal samples by sequencing 16S ribosomal RNA. Adherence to the diet was assessed using the Mediterranean Diet Serving Score (MDSS), in which higher scores indicate greater adherence to the diet.28
Prior to the intervention, the MDSS index was significantly lower in patients with IBS-C compared with controls (p>0.0001), and food diaries showed that patients with IBS-C consumed significantly more protein (p=0.0229) and less carbohydrate and fibre (though these were statistically non-significant).
At the end of the intervention, the MDSS index had significantly increased in patients with IBS-C compared with baseline scores, indicating patients’ adherence to the nutritional protocol (p<0.0001). The relative proportions of proteins, lipids, carbohydrates, and simple sugars, and the quantity of fibre consumed, were significantly closer to the nutritional protocol at the end of the intervention than at baseline (p=0.0199).
Analysis of faecal microbiota showed an increase in Bacteroidetes and a decrease in Firmicutes phyla from baseline to the end of the intervention. At the family level, an increase in Bacteroidaceae and decreases in Ruminococcacae, Lachnospiraceae, Clostridiales, and Prevotellaceae were observed.
In terms of IBS symptoms, there was a significant reduction in pre-evacuation abdominal pain (p=0.0004), abdominal pain during evacuation (p<0.0001), and daily frequency of abdominal pain (p=0.0020) at the end of the intervention compared with baseline.
Crucillà concluded that although these are preliminary results, they indicate a significant role for diet in the control of IBS symptoms. It was emphasised that further studies with larger population samples are needed to confirm these data.
Online Education Significantly Improved Physicians’ and Pharmacists’ Knowledge and Confidence in the Management of Reflux Disease
James White
White emphasised that the prevalence of GORD is increasing at a similar rate to obesity and population ageing,29 and that implementing best practice management of GORD is critical to improving patient symptoms and reducing morbidity. White presented the results of an online, video-based, roundtable discussion-format CME activity, which aimed to assess whether online-based CME could improve physicians’ confidence and knowledge regarding the management of GORD.
Gastroenterologists (n=147), primary care physicians (n=880), and pharmacists (n=1,911) watched a video, entitled ‘Best Practices in the Management of Reflux Disease: Experts Discuss Patient Cases’, which addressed the roles of alginate/antacid therapy and proton pump inhibitors in the management of reflux disease. Before and after the video, participants completed a questionnaire consisting of three multiple choice questions to assess knowledge, and one question to assess confidence regarding the management of GORD.
Results showed that watching the video significantly improved the mean percentage of correct responses for gastroenterologists (from 56% to 73%; p<0.001), primary care physicians (from 45% to 66%; p<0.001), and pharmacists (from 46% to 63%; p<0.001). A measurable increase in confidence was reported by 34% of gastroenterologists, 52% of primary care physicians, and 52% of pharmacists (all p<0.001).
White concluded that participation in a CME programme of this type could significantly improve both knowledge of and confidence in best practice management of GORD, and that future education should focus on using a format based on dynamic patient cases.
DISORDERS OF THE PANCREAS
Disorders of the pancreas affect the ability of this organ to biosynthesise and/or secrete sufficient enzymes to digest food in the intestines.30 Disorders that affect pancreatic function include chronic/acute pancreatitis, neoplastic disease, hereditary diseases (e.g., cystic fibrosis), diabetes, and Shwachman–Diamond syndrome.30 Accumulating research has shown an association between microbiome abnormalities and pancreatic disease, but it is not yet clear whether microbial dysbiosis is a cause or an effect of pancreatic dysfunction.31,32
Gut Microbiota Composition is Significantly Altered in Patients with Chronic Pancreatitis
Peter Macinga
There has been increasing recognition that the gut microbiome may play a role in the modulation of pancreatic disorders.33-38 For example, a faecal microbiota-based classifier has been identified as a potential diagnostic biomarker for pancreatic ductal adenocarcinoma,33 pancreatic exocrine function has been associated with changes in the abundance of specific faecal microbes,34 an altered intestinal microbiota has been identified in patients with chronic pancreatitis,35 and antimicrobials secreted by the pancreas have been shown to play a role in shaping the gut microbiome in mice.36
Macinga presented the results of a study that aimed to characterise the composition of the gut microbiota in 51 patients with chronic pancreatitis and 41 age- and sex-matched healthy volunteers (controls). Stool samples were collected, and the V3 and V4 regions of the 16S ribosomal RNA gene were sequenced to discriminate bacterial species.
The diversity of the faecal microbiome within samples (α-diversity) was similar across both patients with chronic pancreatitis and controls, though diversity tended to be lower in the former. Principal component analysis showed that the faecal microbiome in patients differed from that of controls (β-diversity). Multivariable analysis identified significant differences between groups at the phylum (p=0.035), order (p=0.023), family (p=0.013), and genus (p<0.001) levels. Nine phyla were identified with a significantly different mean abundance between controls and patients with chronic pancreatitis, including Firmicutes (32.0% versus 26.0%) and Proteobacteria (0.1% versus 2.5%). Eighteen genera had a significantly different abundance between groups. Genera enriched in patients with chronic pancreatitis versus controls included Eggerthella, Lachnoclostridium, Escherichia, Shigella, and Enterococcus, all of which are mucin degraders that may promote damage to the gut barrier and trigger systemic inflammation. Genera depleted in patients with chronic pancreatitis versus controls included the short-chain fatty acid-producing bacteria, Christensenellaceae_R.7_group, Lachnospiraceae NK4A136_group, Coproccocus, Clostridia_ UCG.014, and Erysipelotrichaceae_UCG.003.
Macinga concluded that the composition of the faecal microbiota is significantly altered in patients with chronic pancreatitis, with a shift towards a pro-inflammatory setting and compromised intestinal barrier function.
The Use of a Patient Support Programme for Patients with Pancreatic Exocrine Insufficiency in Sweden to Improve Usage and Adherence to Treatment
Joakim Svahn
Svahn explained that the objective of PERT in PEI is to deliver sufficient enzymatic activity into the duodenum with a meal to restore nutrient digestion and support absorption.39
PERT is available in capsules or tablets for oral use, and the appropriate timing of each dose with meal consumption is critical.40 Svahn stressed that it is important for patients to understand how PERT works to optimise compliance and administration of the medicine, and that educational programmes and tools such as PSPs may help in this respect, improving PEI management and clinical outcomes.
Svahn presented data from a survey that aimed to assess the benefits of a PSP service available to all patients who are prescribed PERT in Sweden. Patients enrolling in the PSP are required to provide consent through a dedicated website, and all data gathered are confidential and are deleted if a patient withdraws from the programme. Participation is free and does not affect other healthcare provided to the patient. Participation in the PSP extends over a 36-week period and involves a survey at Weeks 1 and 26, two phone consultations with a trained nurse, and additional emails and text messages to remind the patients to take their prescribed treatment.
A total of 374 patients enrolled in the PSP between June 2016 and March 2022. Fifty-seven percent of respondents were female, 19% had chronic pancreatitis, 15% had pancreatic cancer, and 13% had undergone gastric surgery. Of those patients who were prescribed PERT, 28% received the prescription from a surgeon, 24% from a general practitioner, and 18% from a gastroenterologist.
As of 20th March 2022, 116 patients had completed the second PSP survey. Svahn noted a trend towards improved bowel function over time, with an average composite score (rated on a 10-point scale and including symptoms of pain, discomfort, stool frequency, and stool consistency) of 5.9 at Week 1 (Survey 1) and 6.7 at Week 26 (Survey 2). A slight increase was observed in the mean number of times per day that PERT was taken, from 3.7 times per day at Week 1 to 3.8 times per day at Week 26. Most respondents (78%) reported that participation in the PSP had a positive impact on the management of their PEI.
Svahn concluded that the use of a PSP programme in a Swedish population with PEI represents a valuable aid for patients to self-manage the administration of their prescribed PERT.








