Meeting Summary
Given the progressive nature of Crohn’s disease (CD), Prof Panés made a case for timely intervention in at-risk patients to achieve the ultimate goal of slowing disease progression. Prof Peyrin-Biroulet looked at the more recent treatment target of endoscopic healing and reviewed the positives and negatives of the current endoscopic indices to measure disease activity. Prof Lees then provided an overview of the clinical trial programme and real-world data of vedolizumab, a gut-selective α4β7 integrin inhibitor.
The Case for Early Intervention in Crohn’s Disease
Professor Julián Panés
Aiming to Stop Disease Progression: What is the Right Target?
CD is progressive in nature and so, to avoid irreversible damage and achieve the ultimate goal of slowing down disease advancement, timely intervention is considered essential.2,3 Mucosal healing can be used as a surrogate target for slowing disease progression, as shown by evidence from a Norwegian population cohort of CD patients.4 This study highlighted that mucosal healing after 1 year of treatment was associated with a decreased risk of surgery in subsequent years, compared with patients who had not achieved mucosal healing after 1 year.4 Therefore, once mucosal healing is achieved, the risk of disease progression and the requirement for surgery should be significantly reduced, resulting in a more benign disease course, as Prof Panés explained.
However, mucosal healing in CD may not always be sufficient in Prof Panés’ opinion. A study in Barcelona, Spain, aimed to evaluate the use of colonoscopy compared to magnetic resonance imaging (MRI) as a predictor of the need for resection surgery in patients with CD.5 The results showed that severe endoscopic lesions did not serve as a predictor of resection surgery, whereas transmural lesions in the form of stenosis or intra-abdominal fistulas at MRI were associated with an increased risk of surgery in patients with CD.5 Therefore, transmural healing in CD might be more important than mucosal healing. The evidence from this study contributed to the evolution of therapeutic goals in CD, which, in Prof Panés’ opinion, should include transmural healing.
Optimising Treatment with Current Therapies
Focussing on treatment options, early intervention in CD with biologic-based therapy has been shown to improve patient outcomes. The REACT study6 demonstrated that patients who underwent earlier intervention with combined immunosuppression including an anti-tumour necrosis factor alpha (TNF-α) agent required less hospitalisation, less surgery, and had fewer complications than those who underwent conventional management. Prof Panés therefore emphasised that it is possible to optimise the use of current therapies to achieve better results for patients.
Treatment and therapeutic targets should also be individualised to the characteristics of the patient and the stage of the disease; in patients with milder forms of the disease who have not developed complications such as strictures or fistulas, a step-up approach may be the most appropriate to avoid intensive therapy and the adverse events associated with it. On the other hand, in patients with more progressive forms of the disease, early intensive therapy may be more effective in reducing complications and the need for surgical intervention.
Close-Up on Endoscopic Healing as a Treatment Goal: What Do We Need to Know?
Professor Laurent Peyrin-Biroulet
Target Definitions Used in Clinical Trials
To define appropriate treatment targets for CD, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative came to the evidence-based consensus that a combination of clinical remission and endoscopic remission should be the target. Clinical remission is defined as the resolution of abdominal pain and normalisation of bowel habits, while endoscopic remission is defined as the absence of ulceration at ileocolonoscopy. When endoscopy cannot adequately evaluate the inflammation, the resolution of inflammation as assessed by cross-sectional imaging is a target.2,3
The definition of endoscopic healing in CD trials has evolved in recent years. Until 2009, endoscopic healing was determined by a physician’s assessment using empirical definitions describing ulcers, erythema, or pseudopolyps. The definition then evolved to the absence of ulceration. Although this is now widely used as a primary endpoint in randomised controlled trials,7 there are limitations to this definition because it does not account for remaining lesions, such as erosion, erythema, or oedema, and it is not sensitive to degrees of change.7
Scores for Measuring Disease Activity
To standardise clinical practice, an objective scoring system is needed that is practical in the real world. Endoscopic scoring systems have been developed to evaluate disease activity, including the Crohn’s Disease Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for Crohn’s Disease (SES-CD); however, their usefulness has not been fully assessed.
The CDEIS score was the first validated endoscopic score for CD. Preselected lesions were recorded on a standard form in different segments of the colon. Stepwise multiple regression was then performed to create an index that correlated with the endoscopist’s estimate of lesion severity. This index was shown to be valid in subsequent studies and is therefore useful for those endoscopists who are aware of the data collection procedure in the follow-up of patients, especially in clinical trials.8
The SES-CD was developed later to be a more practical and simple alternative to the CDEIS score, and included only characteristics that contribute to symptomatology, such as scores for ulcer size, ulcerated surface, affected surface, and luminal narrowing.9 The CDEIS score is still regarded as the gold standard and is moderately responsive to changes in endoscopic disease activity;10 however, the SES-CD is simpler than CDEIS10 and may have a greater responsiveness to change in disease activity.11 Both CDEIS and SES-CD are suitable for central reading in clinical trials.7
What is the Right Target to Predict Patient Outcome?
Today, many CD trials use different arbitrary and unvalidated definitions of endoscopic healing and/or response. A French study found that when the strict definition of mucosal healing (CDEIS=0) was achieved, it was associated with better clinical outcomes, including a lower risk of relapse and intestinal resection.12 However, it is hard to achieve a CDEIS score of zero with drugs that are currently available. This was highlighted by the CALM study,13 an open-label, multicentre study that evaluated two different treatment algorithms in patients with moderate-to-severe CD, with one tight control treat-to-target arm and one conventional clinical management arm that mirrored the treatment in clinical practice at the time the study was designed. The results showed that the percentage of patients on the treat-to-target approach who achieved complete endoscopic remission, defined as CDEIS=0, was lower (18%) than those who achieved endoscopic remission (46%), defined as CDEIS <4.13
During the induction phase of treatment, endoscopic response as a treatment goal is a valid predictor for patient outcomes, whereas the goal of endoscopic remission is more important during follow-up with the patient. A post-hoc analysis of the SONIC study14 of biologic and immunomodulator-naïve patients in CD aimed to determine the best definition for endoscopic response on the SES-CD and CDEIS indices. It was found that endoscopic response, defined by a decrease from baseline in SES-CD or CDEIS of ≥50% at Week 26, was useful to predict the number of patients in corticosteroid- free clinical remission at Week 50.14
To standardise the practice, the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) recommend an endoscopic response definition of >50% decrease in SES-CD or CDEIS, and an endoscopic remission definition of a SES-CD score of 0–2.7 However, both the endoscopic response and endoscopic remission definitions require prospective testing in CD trials.7
Usage of Scores and Targets in Clinical Practice
In contrast to randomised controlled trials, in clinical practice endoscopy scores are often not used for patients with CD because clinical activity is regarded as more relevant for gastroenterologists to base their therapeutic decisions on.15 This was confirmed by the results from a survey that gathered data on the management of inflammatory bowel disease (IBD) and showed that endoscopic scores are only used in 11% of patients with CD.16 In conclusion, it is important to establish an objective scoring system to measure endoscopic healing. The CDEIS or SES-CD indices have enabled results to be compared across studies and the effort should be continued to standardise the practice using clear definitions of endoscopic response and mucosal healing. For real-world practice, disease management needs to be based on an objective scoring system and, by improving endoscopic reports and using the CDEIS score or the SES-CD, this could be achieved in the future.
Meeting Evolving Treatment Goals with Gut-Selective Therapy
Professor Charlie Lees
How to Meet the Targets with Current Therapeutic Options
Following the previous talk on how to define and measure treatment targets, Prof Lees focussed on how these targets could be met with currently available therapies. He reminded the audience that CD is characterised by periods of flares and periods of remission, and that the periods of flares can result in accumulation of bowel damage as the disease evolves over time.17 This leaves a window of opportunity to achieve the best results for patients when treating early in the disease course. When treating patients to the predefined targets, it is important to keep value-based healthcare in mind to achieve cost-effective solutions. Prof Lees argued that the only way to control rising costs is to strive to improve patient outcomes efficiently, which requires investment in quality of care that is safe, appropriate, and effective.18
Important data showing the benefit of a treat-to-target algorithm came from the CALM trial.13 The results showed that when applying intensive, highly controlled treat-to-target treatment with an anti-TNF-α-based strategy, mucosal healing (defined as CDEIS <4 and no deep ulcers) was achieved in around 46% of patients at Week 48 of treatment; in the conventional clinical management arm, around 30% of the patients achieved mucosal healing.13 However, this still leaves over half the patients requiring alternative drugs to help them reach remission, as Prof Lees stated. Currently approved biologic therapies for moderate-to-severe CD comprise TNF-α inhibitors (infliximab,19 adalimumab20), interleukin-12/23 inhibitors (ustekinumab21), and the gut-selective anti-α4β7 integrin (vedolizumab1). Thus, there is a variety of biologics that can be used to treat patients with moderate-to-severe CD and it is important to choose the right timepoint to assess if the biologic is working for the patient.
Gut-Selective Treatment: Short-Term and Long-Term Goals
Looking at how the gut-selective vedolizumab can be used to achieve the STRIDE targets, relevant evidence is available from a post-hoc analysis of GEMINI 2 and 3 pooled data. Results showed that in an anti-TNF-α-naïve population, 48.9% of patients treated with vedolizumab achieved clinical remission (CD Activity Index ≤150) at 52 weeks (versus 26.8% with placebo), and in the overall population, including patients with failed anti-TNF-α treatment, 37.7% of patients achieved this (versus 21.6% with placebo).22 In a GEMINI 2 post-hoc analysis looking at the onset of symptomatic improvement, vedolizumab showed a significant response in a composite score of reduction in abdominal pain and liquid or soft stools compared with placebo at Weeks 2, 4, and 6. This demonstrates the relatively fast alleviation of symptoms in some patients treated with vedolizumab, notably those without prior exposure to anti-TNF-α agents.23 The same trend was seen in the overall population, but less markedly.23
However, there are patients whose response will take longer, and thus further assessments will be necessary, including endoscopic assessment of mucosal healing, as the STRIDE targets suggest. Long-term data on mucosal healing with vedolizumab came from the GEMINI LTS cohort where the primary objective was to evaluate the long-term safety profile of vedolizumab. A retrospective chart review of anti-TNF-α refractory CD patients (n=24) with a median vedolizumab treatment duration of 3.2 years showed that 29% of patients had complete mucosal healing (no ulcers as recorded by a physician’s assessment), 38% had partial healing (marked improvement), and 33% showed no healing.24
Real-world data provide further evidence on mucosal healing with vedolizumab; in a study by Vivio et al.,25 30% of CD patients showed complete mucosal healing, defined as an SES-CD of zero, and 52% showed endoscopic improvement at 22 weeks. This was also observed by the Amiot et al.26 study, which showed that 30% of CD patients treated with vedolizumab had no ulcers between 30 and 54 weeks of treatment duration, and the US VICTORY consortium, where 58% of patients had no ulcers or erosions at 52 weeks.27 Currently, there is ongoing research that will provide more breadth to the available data on mucosal healing with vedolizumab.28
The Goal of Maintaining Remission
The current data show that it is possible to achieve remission and mucosal healing with vedolizumab, but a focus on maintaining remission is also paramount. With anti-TNF-α therapy, a loss of response over time can be expected by ≥50% of patients at 12 and 24 months.29-31 Additionally, there remains a safety signal with anti-TNF-α therapy, albeit relatively modest, when looking at predictors of serious infection. For example, in the TREAT registry,32 infliximab treatment was identified as an independent predictor of serious infection, although prednisone, narcotic analgesic therapy, and ongoing moderate-to-severe disease activity were identified as higher risk factors.
Data on lasting clinical remission with vedolizumab can be derived from the GEMINI LTS cohort; participants in this long-term safety study included patients who completed the maintenance phase of the GEMINI 2 trial and continued to be treated with vedolizumab every 4 weeks (see disclaimer). Of them, 74% were in clinical remission at 52 weeks and 89% (n=54/61 observed cases) of patients achieved clinical remission after approximately 5 years of cumulative exposure. Thirty-seven percent of patients were in remission when the stricter analysis was applied, where all patients without available 5-year-data were counted as non-responders.33,34
The Safety Profile: Of High Importance for Long-Term Treatment
For a long-term treatment, the safety profile is of special importance. With a total exposure of 4,811 patient-years, a significant amount of safety data is accumulating for vedolizumab. Data pooled from six clinical trials showed that there was no increase in adverse events in patients treated with vedolizumab versus placebo in a 5-year safety analysis and no cases of progressive multifocal leukoencephalopathy were reported (Figure 1).35 This was confirmed by a meta-analysis of real-world safety data, which evaluated 2,857 vedolizumab-treated patients and reported adverse event rates consistent with the data from the clinical trials.36 As of August 2017, vedolizumab has 143,127 patient-years of post-marketing exposure worldwide, a number that has doubled from the previous year.
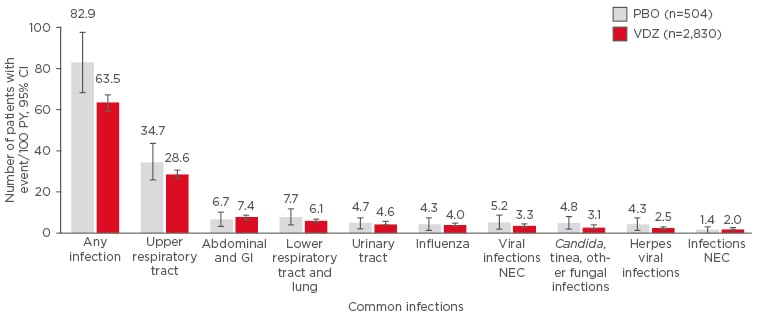
Figure 1: Data pooled from six trials (total exposure: 4,811 patient-years) show that there was no increase in adverse events in those patients treated with vedolizumab versus placebo in a 5-year safety analysis.
A common infection was defined as ≥2 patient events/100 PY in the VDZ group.
CI: confidence interval; GI: gastrointestinal; NEC: not elsewhere classified; PBO: placebo; PY: patient-years; VDZ: vedolizumab.
Adapted from Colombel et al.35
In conclusion, the treatment goals for CD are evolving to a composite target of clinical and endoscopic remission, with the main aim of slowing disease progression. By optimally using the current drugs on the market, the aim is to avoid long-term complications. This involves identifying high-risk patients early in their disease course and treating them with an appropriate biologic that can achieve and maintain clinical remission. Vedolizumab, a gut-selective biologic, has been shown to achieve and maintain clinical remission in the long-term, with early symptomatic improvements and mucosal healing shown by exploratory/real-world data. Vedolizumab also has a favourable safety profile, as demonstrated in 5-year pooled clinical and real-world studies.








