Meeting Summary
The emergence of anti-TNF biosimilars has had significant implications for the biologic treatment of inflammatory bowel disease (IBD). Significant cost savings provide an incentive for healthcare providers to encourage the prescription of biosimilars instead of reference products. However, patients may have concerns about the switching process, the reason for the switch, or the biosimilar itself, and it is important for healthcare professionals (HCP) to take these into account to enable an informed, shared treatment decision.
The aim of this symposium was to understand treatment of IBD from the patient’s perspective, especially when switching treatment to a biosimilar product. Beginning with a review of the current and future treatment landscapes, the implications of the increasing availability of biosimilars were discussed. The role of HCP in communicating information about the switch was explored by the multidisciplinary faculty who also compared switching practices at their own treatment centres and shared best practices. Alongside videos of interviews with patients who had undergone a switch to a biosimilar, a patient advocacy perspective was provided by Ms Luisa Avedano, CEO, the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA).
Introduction
A highly interactive symposium, including pre-recorded interviews with patients, was chaired by Prof Geert D’Haens. Participation by the audience was strongly encouraged through live polling and submission of questions to the faculty throughout the meeting.
In recent years, biologics have become a cornerstone in the management of IBD. As reference product patents expire, biosimilars join the treatment armamentarium, adding an exciting and relevant dimension.1
Overall healthcare costs are substantial in IBD, with an estimated €1,625 spent in Crohn’s disease and €595 in ulcerative colitis per patient every 3 months, much of which is spent on anti-TNF agents (64% and 31% of the total cost in Crohn’s disease and ulcerative colitis, respectively).2
Between 2007 and 2020, the introduction of biosimilars has been estimated to offer potential cost savings of between €12 and 33 billion in the European Union (EU).3 Both direct and indirect benefits are offered by biosimilars. When biosimilars enter the market at reduced prices, this is usually accompanied by a reduction of the price of the reference product. This reduced cost burden in one product allows money to be reinvested into healthcare systems, which might enhance patients’ access to effective treatments.3-5
While comparability between approved biosimilars and their reference products in terms of safety, efficacy, immunogenicity, and pharmacodynamics has been shown in randomised controlled trials,6 patients may have reservations about being prescribed therapeutics other than the reference biologic or being switched to a biosimilar. A lack of confidence in treatment, driven by a lack of communication and shared, informed decision-making between the HCP and their patient in preparation for a switch, may lead to subjective loss of response or side-effects.
Multidisciplinary Approach to Managing Inflammatory Bowel Disease
Following Prof D’Haens’ introduction, the roles of different members of the healthcare team in the pre-switching process were discussed by the faculty. Prof Bouhnik advised that switching should only be done based on a shared decision between the physician and their patient. During the initial switching discussion, it is essential to be clear that the proposed medication is not a new drug or mechanism of action, but is, as Prof Bouhnik noted, a: “similar: the word is important.” Following this preliminary conversation, an IBD nurse would be the main point of contact. In Prof Bouhnik’s opinion, physicians are often confined by the time pressures of a busy clinic, and expert nurses are more adept at conversing with patients about switching following the initial consultation.
HCP responding to a pre-symposium questionnaire said an initial consultation to verbally discuss the switch procedure could last from 15 to >30 minutes. Additional information was provided in educational leaflets or letters, and patients were directed to educational websites. After a switch, patients were monitored and a follow-up appointment was scheduled <1 month to 3–6 months post-switch.
Ms Maria de Jong described the role of the nurse in pre-switch communication. Patients are likely to think of questions and concerns following the initial conversation with the treating physician, which they did not articulate during this consultation. It is, therefore, important to have a member of the healthcare team whom patients are able to contact readily to discuss these. The expert nurse can fill this role, with part of their time being set aside for telephone consultations with patients.
Multidisciplinary Team Communication: Healthcare Professionals
Prof D’Haens noted that the Netherlands, and, within it, the Academic Medical Centre (AMC), were among the earliest locations to adopt biosimilars, with their prescription being encouraged for economic reasons. There was, therefore, little information from other units on how best to communicate with patients about the switch process. Ms de Jong explained that a team approach was decided upon, which included gastroenterologists, IBD nurses, day care centre nurses, a PhD candidate, and the patient. Ideally, the physician talks to the patient about biosimilars in person, but occasionally a telephone consultation is required for practical reasons. Discussions last for approximately 10 minutes, during which the physician provides information and seeks informed consent for a switch to a biosimilar. The physician aims to provide relevant information regarding the switch in a positive and encouraging manner, but ultimately the patient is responsible for making an informed decision whether to undertake a switch. Ms de Jong also explained that IBD nurses at the AMC clearly explain to patients that they can switch back to the reference product after commencing the biosimilar. In addition, a flyer was developed at the AMC that could be distributed prior to the consultation to provide the patient with some context of the proposed switch or, ideally, following a consultation to provide the patient with further written information.
Prof Atreya described the initial consultation in which patients are introduced to biosimilars, stressing that the most important message to convey was: “more of trust and more of emotion”; patients successfully treated with reference biologics may be averse to changing a therapy that has improved their quality of life or even resulted in disease remission. Ms Luisa Avedano, CEO, EFCCA patient associations (PA), reported that, despite awareness of biosimilars being lower and communication strategies regarding switching being in an early stage at the time of a 2014–2015 survey, a strikingly low figure of 11.7% (n=383) of patients agreed with the statement that they: “trust their pharmacist or treating physician” if they prescribe or deliver a biosimilar following treatment with a reference product.
Prof Raja Atreya highlighted the importance of being open about economic reasons for switching from both a perspective of trust and one of practicality, noting that with sufficient numbers of patients switching to biosimilars, further HCP could be recruited to the unit, leading to a better standard of care through increased availability of staff: “They could really see the waiting times reduced and this was an important factor to motivate them,” Prof Atreya explained. Prof D’Haens reported that patients may be encouraged to switch if they consider that cost savings could be used to pay for more effective treatments for other conditions where less expensive therapies are not available.
After the possibility of a switch is introduced by the physician, additional information can be provided by IBD nurses via face-to-face consultation, via telephone, or as printed material. Prof Atreya, Ms de Jong, and Ms Avedano all noted that patients are interested in the results of switching studies. “We have more information, and we can use this information,” said Ms de Jong, indicating that clinical data can be shared with, and explained to, the patient by the physician and nurse to engage with them, build trust, and ultimately make a well-informed, shared decision.
HCPs’ communication with their patients can also involve directing them to other organisations. Ms Avedano iterated that EFCCA is encouraging HCP to direct newly diagnosed patients to PA. Ms de Jong agreed with the importance of this, confirming that the AMC treatment pathway included making patients aware of PA. Later in the symposium, Prof D’Haens referred to the rheumatology department at the AMC, where patients treated with reference biologics were switched to biosimilars under the care of physicians and specialist nurses, with an information package that was assembled by both HCP and representatives from PA and suggested that this may be an effective way to communicate information most appropriately to patients.
Multidisciplinary Team Communication: Patient Perspective
The first interview video saw two patients describing how and when they were first told about biosimilars. One patient, treated at AMC, was told about biosimilars and a potential switch during a routine appointment. The other patient, treated at Paris-Diderot University, Paris, France, was introduced to biosimilars at the day hospital during a reference infliximab infusion appointment, having a 20-minute conversation with his physician about a change in treatment. Initially he: “did not know what to expect.” He had the opportunity to ask questions to both the physician and nurses. Following this appointment, he conducted some research on the internet to find more information and reported he: “saw that it was done in other areas of health.” Discussing biosimilars with his family suggested that they had: “no more worries” than him. He found the transition to a biosimilar straightforward; both the reference product and new treatment were administered as infusions, so the change was not significant. He reported that he was: “waiting to see the effects and, in fact, there is not much of a difference.”
Regarding communication between patients themselves about the switching process, Ms de Jong reported that: “patients are also talking with each other about their experience, and if it’s positive it is easier.” Conversations can be held at day care centres and infusion appointments, and that discussion is ongoing on social media platforms. PA, such as EFCCA, also facilitate this dialogue.
Initiating the Switch: Pathways and Programmes
Both Prof Bouhnik and Prof Atreya responded to a question from the audience asking whether they deemed any patients ineligible for a switch and both replied that, in their opinion, all patients could be initiated on a biosimilar, but that switching back is a possibility if adverse effects (AE) are subsequently experienced.
Prof Atreya reported that at his centre, the University of Erlangen-Nürnberg, Erlangen, Germany, over a 2-month period, 100% of patients with IBD on reference infliximab (N=˜200) had their treatment switched to a biosimilar. This switch was mandatory; patients not accepting the change in medication would: “have to look for another infusion centre.” However, only one patient did not consent to the change, and they returned to the Erlangen-Nürnberg clinic after one infusion at another treatment centre. Notably, this patient reported that she returned primarily because she found the nursing care to be of lower quality at the new centre. Prof Bouhnik referred to a study which reported results from a French treatment centre: 86 patients with IBD receiving treatment with reference infliximab were offered a switch to biosimilar. Of these patients, 47% initially refused the switch but, of these, 78% agreed to participate in an education interview with a nurse. Following this, a total of 68% of patients finally accepted the switch.7 These instances highlight the importance, from the patient’s perspective, of the role of the nurse in the multidisciplinary team (MDT), especially in providing education in a clinical setting.
Key Insights from Patient Associations: The Patient Representative Perspective
EFCCA represents 36 IBD PA from 35 countries and engages, on average, between 10% and 20% of patients with IBD within each country. Ms Avedano said that EFCCA was committed to “making the invisible visible” regarding IBD, and noted wide variation in switching practices between countries and, in some instances, regional differences within the same country. This variation, alongside a general lack of information about biosimilars, could make patients uncomfortable as they see: “a general picture of the situation that does not necessarily mirror reality in every country.” To investigate patients’ level of knowledge and perspectives about biosimilars, EFCCA developed the Biologics and Biosimilars Online Survey (BAB), which was conducted from 2014–2015.7 Across Europe, 1,181 patients completed the survey. Just 38.0% of surveyed patients had heard of biosimilars and only 25.2% of this group of patients familiar with biosimilars reported having no concerns about them.8
Results of the BAB study prompted EFCCA to organise a series of advocacy and educational workshops to address the perceived lack of information available to patients.9 These included not only patients with IBD treated with biologic therapeutics, but also patients with other immunomediated conditions who were able to offer insights into their treatment pathways and the switching process. Across disease groups, patients reported room for improvement in communication with physicians and also the crucial role of specialist nurses in treatment pathways. Between countries, there was significant variation in the availability of specialist nurses within the MDT. Prof Atreya and Prof Bouhnik said that the situation in Germany and France, respectively, was that specialist nurses were beginning to be introduced to the IBD MDT, and that the practice was growing but not currently universal. Ms Avedano reported a lack of understanding amongst policymakers with whom EFCCA engaged following these workshops to raise the concerns identified by patients, including a lack of awareness of IBD, treatment pathways, and costs (both direct and indirect) associated with the condition. Reports from the EFCCA workshops following the BAB have suggested improvements in communication since, but there is significant scope for improvement across Europe.
Following a Switch Phase and Subsequent Follow-Up: Patient Perspective
The patient treated at AMC reported that: “the communication went well and the information provided to me was great.” The patient was in close contact with the treating physician who gave guidance during and after the switch itself. Alongside this, the patient was: “always able to ask questions to the IBD nurse… which made the transition a very pleasant experience.” The patient responded that he felt as good as he did before the switch and suggested that, to improve patient care following a transition to a biosimilar, communication between patients and HCP should be made as easy as possible, so that any questions and concerns can be addressed quickly. Figure 1 illustrates roles and interactions within the MDT to aid a successful switch.
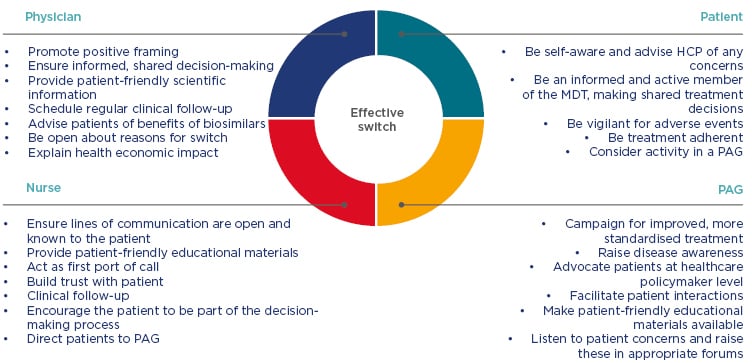
Figure 1: Roles and responsibilities of key participants for a successful switch.
HCP: healthcare professionals; MDT: multidisciplinary team; PAG: patient advocacy group.
Follow-Up and Faculty Advice: Optimising Post-Switch Care
Prof Bouhnik described the ongoing PERFUSE study,10 designed to gain insights into post- switch patient perspectives by collecting data on post-switch drug survival rates. PERFUSE is a long-term, prospective, observational, multicentre cohort study investigating SB2 (an infliximab biosimilar) discontinuation in 1,500 French patients who were switched from the reference product in five autoimmune conditions. The patient perspective is also being explored via measurement of patient-reported outcomes, treatment perceptions, and satisfaction regarding the information about biosimilars that was provided to them.
Ms de Jong described a study, in which the AMC participated, that followed patients with IBD in remission for 16 weeks. Pharmacokinetics (PK) and disease activity (via the simple clinical colitis activity index or Harvey–Bradshaw index) were measured, along with antidrug antibody formation, AE, and patient-reported outcomes. In 88 patients with IBD in remission (29 at AMC) who were treated on reference infliximab for >30 weeks, a subsequent switch to a biosimilar was found to be safe and well tolerated.11
Prof D’Haens asked the faculty how they responded to patients who were switched to biosimilars reporting that the new product was ineffective or causing AE. Ms de Jong confirmed that the MDT would work to establish the problem, including carrying out PK investigations, such as trough levels, but that physiological reasons for inefficacy or AE may not be found. Prof D’Haens mentioned that subjective factors can be involved in patients wanting to switch back, citing the nocebo effect, and that clinical trial results and his experience indicate that a number of patients do switch back to the reference product after commencing a biosimilar.12 Prof Bouhnik confirmed that patients had the right to switch back to reference products following a change to biosimilar treatment in France and mentioned that the nocebo effect was a noted problem, while Ms de Jong and Prof D’Haens reported a switch back rate of <5% at the AMC. Prof Atreya presented Harvey–Bradshaw index and partial Mayo scores of patients with ulcerative colitis and Crohn’s disease, respectively, up to 24 weeks post-switch from reference infliximab to SB2, which showed no statistically significant difference over the study period.13
A New Approval: A Different Story for Adalimumab?
The experiences described so far relate to infliximab administered by intravenous infusion but, with the recent market authorisation of adalimumab biosimilars (self-administered subcutaneously), the faculty members were asked for their predictions of how the two drugs would compare in relation to switching.
Prof Bouhnik reported that there were differences in the acceptance rate of patients agreeing to switch from infliximab and adalimumab reference products to their biosimilars; in his clinical experience up to 7 out of 10 consecutive patients would not accept a biosimilar to reference adalimumab. Although real-world evidence for the safety and effectiveness is convincing for patients, the timing of the introduction of adalimumab biosimilars means that these data have not been available until recently, accounting for this reluctance to switch. An audience member asked whether the faculty expected adalimumab switch programmes to be as simple as those for infliximab. Prof Bouhnik thought that they would be: “much more difficult… the nocebo effect will be a major problem,” due to self-injection administration with a number of different devices available. Prof Atreya added that the increased logistic effort of measuring trough levels for adalimumab makes undertaking PK studies in the real world more difficult, but that experience gained in infliximab switching programmes would be valuable in facilitating the adalimumab switching procedure.
Prof D’Haens noted that, while being similar in terms of the active biologic, adalimumab biosimilars were different in terms of excipients included in the complete formulation, administration devices, stability, and shelf-life.
Furthermore, Prof D’Haens addressed the importance of biologics stability.14 Indeed, in a study including 255 patients, only 6.7% of the patients stored all biological disease-modifying antirheumatic drug packages within the defined Summary of Product Characteristics-recommended temperature range. It was noted that SB5 (an adalimumab biosimilar) has an approved cool chain shelf life of 3 years, compared with 2 years for the other adalimumab products, and that data supporting the stability of SB5 at room temperature (25 °C) up to 28 days have been recently published (and, since the symposium, approval for storage at temperatures of up to 25 °C for a period of up to 28 days has been granted by the European Medicines Agency [EMA]),15 which could have implications for the daily activities and lifestyles of patients.16 Prof D’Haens noted that some preparations of adalimumab are more appropriate than others for individual patients, depending on formula, stability, and other product characteristics. Symposium attendees were asked a live polling question: “Were you aware of the differences between adalimumab products, in particular regarding stability?” and 63.3% responded that they were familiar with the subtle variations in preparations not relating to drug activity or immunogenicity.
Conclusion
The patient’s perspective is paramount in the IBD treatment pathway, especially when considering switching to a biosimilar from a reference product. From the initial consultation in which the patient is introduced to biosimilars to all interactions following a switch, communication is key. Easily contactable specialist nurses in the MDT are valued greatly by patients, and advocacy groups are campaigning for increased access to MDT members, along with more consistency in messages and practices regarding biosimilars to inspire confidence in their uptake. The process of switching patients treated with adalimumab may present new challenges. Nocebo effects may have a greater impact due to the fact that these products are self-administered. It will require thoughtful communication from HCP to transfer confidence to patients, but this could lead to increased initial uptake rates and reduction of the nocebo effect.17 With well-considered switch programmes, patients can be confident in their therapeutics with beneficial results for healthcare economics.
The importance of communication was confirmed following the symposium, when faculty members were interviewed individually and asked for advice they would offer to patients preparing for a switch. Prof D’Haens said that: “the process starts with information, but the patient may come back with questions, and then there needs to be somebody available to answer, and I think we underestimate that as physicians.” Ms Avedano highlighted the importance of involving the patient in treatment decisions by providing them with educational materials appropriate to their understanding and stressed the importance of having a well-functioning MDT, including specialist nurses.
Prof Atreya said that: “you really have to be a partner,” asking the patient whether, from their perspective, they noticed: “any difference in quality of life.” “We can assess the clinical disease activity but what is much more important is what the patient feels,” he concluded. Ms de Jong emphasised a proactive communication strategy, with the treatment centre contacting the patient, while Prof Bouhnik recommended assessing efficacy and immunogenicity of the new treatment using biomarkers or therapeutic drug monitoring and: “especially, to give them the possibility to contact the team whenever they want.”