Meeting Summary
This symposium took place during the 15th congress of the European Crohn’s and Colitis Organisation (ECCO). Dr Raine began the meeting by explaining that all may not be as it seems when we consider the use of corticosteroids for inflammatory bowel disease (IBD). He indicated that despite guidelines recommending the use of corticosteroid-sparing therapies, steroids continue to be widely used, particularly to treat moderate-to-severe ulcerative colitis (UC). Dr Raine presented recent evidence from the UK indicating that increased monitoring of steroid treatment in clinical practice can reduce corticosteroid exposure and excess. Prof Dotan explained that roughly half of excess steroid use was avoidable, and that hidden figures for corticosteroid use indicate that prescribing behaviours do not match current guidelines. She explained that increased patient assessment is needed to avoid prolonged and repetitive corticosteroid treatment courses, and that it is crucial to identify how patients are using steroids, and what can we do to improve their health. Finally, Dr Silverberg described the mechanism of action of corticosteroids and the development of steroid-sparing treatments. He explained the importance of steroid-tapering protocols and steroid-free remission (SFR) outcomes in the design of clinical trials and summarised these aspects of recent trials. To conclude the symposium, Dr Raine summarised the key take-away messages from the meeting.
Standard of Care or Caring Standard?
Steroids: The Key to Unlocking Quality Care in Inflammatory Bowel Disease
Doctor Tim Raine
Dr Raine described corticosteroids as the first IBD drugs to undergo clinical trials. A seminal study, one of the first to introduce the concept of a randomised controlled trial, was conducted in the 1950s by Truelove and Witts.1 It established steroids as a tremendously effective induction therapy for patients with active UC and was followed by similar data for Crohn’s disease (CD) 20 years later.2,3
However, it is important to understand that corticosteroids are firefighting tools. There are limitations to their efficacy; for example, they are associated with imperfect outcomes, are not effective as maintenance therapy, and have little to no effect on mucosal healing. Steroids are also associated with significant side effects, including excess mortality.4 Most of the evidence regarding steroid use in IBD comes from studies conducted between 1955 and 2008; there are few recent publications.5
Many professional bodies now recommend that corticosteroids should be used more cautiously. The American College of Gastroenterology (ACG) guidelines for CD state that steroids should be used sparingly, successfully discontinued, and replaced with steroid-sparing agents.6 The ACG guidelines for UC recommend that the goal of therapy should be to obtain and maintain SFR.7 ECCO has issued similar guidelines for CD and UC, with the suggestion that corticosteroid dependency (the inability to wean steroids below a threshold without relapse) or excess (over one course of steroids in a 12-month period) should warrant a steroid-sparing strategy.8,9 The importance of reducing steroid use is also recognised by patient groups: Crohn’s and Colitis UK recognise SFR as a key step on the ideal patient journey,10 and an online Danish study found that steroid-avoidance was an important treatment attribute among people with UC, second only to effective therapy.11
Despite these recommendations and patient preferences, steroids continue to be widely used. There is a lack of global data for corticosteroid use, which makes it difficult to generate guidelines for steroid tapering.12 However, a 2017 USA study reported that 42% of patients with CD were treated with corticosteroids as first-line therapy, and 63% of these patients received two or more cycles.13 In Canada, steroid use for IBD has remained constant, and even increased slightly, over the past 10 years.12
Although corticosteroid tablets are inexpensive, the associated healthcare costs of uncontrolled diseases are much higher when compared to effective maintenance therapy. For example, the USA medical service costs for oral-steroid-treated CD are $27,041, compared to $12,743 for immunosuppressant therapy and $13,179 for anti-TNF therapy, with similar findings for UC.14
A steroid assessment tool (SAT) has been developed in the UK; data generated from its use indicated that 30% of IBD patients in the UK are treated with steroids over a 12-month period, and 50% of these patients are using them at excessive levels or are steroid dependent.15 Patients with moderate-to-severe UC are the most at-risk group for steroid exposure and steroid excess.15 Another UK study found that over a 12-month period, 16.6% of paediatric patients with IBD received oral steroids, and one-third of these had a 3-month course of treatment more than once.16 Corticosteroid excess is more likely to occur with concomitant thiopurine use, and less likely with the use of anti-TNF therapy, multidisciplinary teams, and quality improvement programme participation.17
In the UK, steps are being taken to address the excess use of corticosteroids, including patient information sheets, an IBD toolkit for primary physicians which emphasises the need to liaise with secondary care physicians before initiating steroid treatment, and new standards to indicate the importance of monitoring and auditing steroid use.18-20 Increasingly, steroid assessment is widely recognised as a potential surrogate marker for quality of care standards in IBD.
In summary, despite ECCO guidelines recommending the use of corticosteroid-sparing therapies, as well as patient preferences, steroids continue to be widely used, with cases of moderate-to-severe UC most at risk. Simply asking the question of “how much steroid treatment am I using in my practice?” appears to be associated with a reduction in corticosteroid exposure and excess, as achieved by the introduction of the SAT in the UK.17
Hidden Figures: What is Happening Outside of your Care?
Professor Iris Dotan
Prof Dotan explained that although corti costeroids are undoubtedly important for the treatment of UC and CD, practitioners should also be aware of significant complications associated with their use. In 2011, the American Gastroenterology Association (AGA) issued quality indicators for IBD care, including the prescription of steroid-sparing therapy and bone-loss assessment during treatment to prevent iatrogenic injury.21,22 Prolonged steroid treatment, even with low-to-intermediate doses, results in accumulation of side effects with potentially increasing severity (Figure 1).23
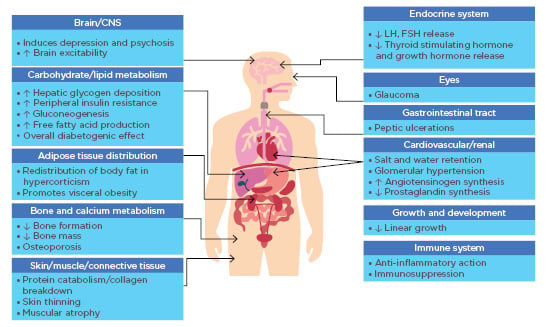
Figure 1: The multifarious consequences of corticosteroid excess. (Courtesy of Prof Dotan)
CNS: central nervous system; FSH: follicle stimulating hormone; LH: luteinising hormone.
Clinicians in both IBD-specialist and community centres in Canada and Germany may be underestimating the level of corticosteroid use in their patients, with community centres more likely to prescribe therapy for >3 months, and less likely to prescribe anti-TNF therapy.24 The 2015 UK SAT audit found that almost 50% of excess steroid use was avoidable, with patients more likely to receive inappropriate courses of steroids from primary care versus secondary care physicians.15
However, it is also possible that some patients refuse corticosteroid-sparing therapy, perhaps out of concern for potential side effects. In one case study, an 83-year-old man with ileal CD had experienced several exacerbations over the previous few years which had been successfully treated with budesonide. Despite significant osteoporosis and endoscopy-confirmed active disease, the patient refused to consider corticosteroid-sparing therapy. He admitted that he used budesonide for 1–2 weeks every time he experienced increased bowel movements, constituting excessive use. In this case, steroid use was masking the patient’s CD progression. Small changes to practice can make a difference for IBD patients, including routine follow-up with questions about active steroid use, patient information sheets on corticosteroids, and new treatments, patient helplines, and monitoring steroid prescriptions.
In summary, hidden figures for corticosteroid use suggest that prescribing behaviours are not matching guidelines. Assessment is needed to avoid prolonged and repetitive corticosteroid treatment courses. It is crucial to discover how patients are using steroids, and what we can do to improve their health. We need to find out why some patients are self-medicating, and how we can change this to improve patient care.
The Evolution of an Endpoint: A Look into Trials from Past and Present and Changing Approaches to Steroid-Free Remission
Doctor Mark Silverberg
IBD is a hugely complex, heterogeneous disease with multiple inputs and pathways (Figure 2).25-28 The current aetiology hypothesis involves the interaction of epithelial cells with microbiota, causing structural or functional changes in the gut microbiome that interact with genetic factors and lead to unregulated inflammatory processes.
Although there are multiple ways to block this process, such as monoclonal antibodies against cytokines, corticosteroids are appealing because of their broad activity (Figure 2).25-28 Corticosteroids act against multiple inflammatory pathways, and cytokine inhibition is likely to be responsible for the general feeling of wellbeing experienced by patients. The main mechanism of action is through glucocorticoid receptors (GR) on the surface of many cells; these receptors inhibit proinflammatory transcription factors such as NF-κB.
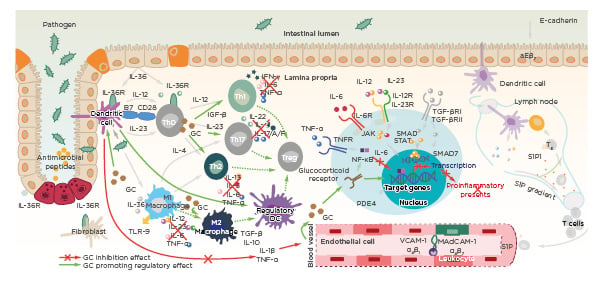
Figure 2: Corticosteroids act broadly targeting multiple proinflammatory pathways.25-28
CD28: cluster of differentiation 28; DC: dendritic cell; GC: glucocorticoid; MAdCAM-1: mucosal vascular addressin cell adhesion molecule 1; S1P: sphingosine-1-phosphate; PDE4: phosphodiesterase Type 4; R: receptor; SMAD: mothers against decapentaplegic homolog; STAT: signal transducer and activator of transcription; TE: effector T cell; TGF: transforming growth factor; Th: T-helper; TLR-9: toll-like receptor 9; Treg: regulatory T cell; VCAM-1: vascular cell adhesion protein 1.
Early trials identified that corticosteroids were effective in inducing remission in active UC and CD compared to placebo.5 However, they are not effective as maintenance therapy, and have little effect on mucosal healing. In one study of patients with CD, corticosteroids achieved remission in 48% of cases (n=109),29 yet after 1 year only half of these cases remained in remission.2 Similar results have been found in other studies.30 Since long-term use of steroids for IBD is associated with significant adverse effects, steroid-sparing therapies are critically important.30 The ideal IBD therapy should focus on healing the bowel, resulting in endoscopic remission. In another study of patients with active CD, corticosteroids achieved clinical remission in90% of patients, but 70% of these had ongoing significant mucosal abnormalities, such as ulceration and erythema.31 This highlights the illusion of remission, whereby patients feel better during treatment, and clinical remission scores reflect this, but the objective outcomes of treatment are not reflecting the goal of endoscopic remission.
The reason why corticosteroids stop working in cases of IBD is not fully understood, but it is likely that a combination of disease severity, dose versus bioavailability, genetic factors such as drug metabolism, and the effects of elevated cytokines in the IBD pathway all play a role. GR have many isoforms, but the most prevalent are GRα and GRβ. GRα has the strongest anti-inflammatory activity, which is achieved through inactivation of several NF-κB pathways, suppressing the transcription of this proinflammatory protein.26 GRβ may have the opposite effect, as it does not bind to glucocorticoids, and has been associated with glucocorticoid resistance in UC.26 Polymorphisms in genes such as the multidrug resistance gene (MDR1) can influence the bioavailability of corticosteroids by modifying the efflux of steroids from cells; it is also possible that this process becomes upregulated during prolonged treatment.26
To achieve our goal of SFR, the outcomes and endpoints defined for clinical trials of new therapies need to be carefully designed. The original IBD studies looked at short-term induction of remission, had no specific corticosteroid-tapering protocols, and did not include SFR as an endpoint. More recently, clinical trial designs have become increasingly sophisticated, with sequential consideration of induction and maintenance, SFR as a secondary endpoint, re-randomised responder maintenance, and in some cases, steroid-tapering schedules during the induction phase.
- CHARM32 (CD) and ULTRA33 (UC) trials of TNF inhibitor, adalimumab, did not include steroid tapering during induction; instead, tapering occurred during the maintenance phase, at the discretion of the investigator.
- COMMIT34 (CD) investigated the efficacy of combined methotrexate and infliximab and included mandated steroid tapering at Week 1, with discontinuation by Week 14. This study showed relatively good SFR rates (>50%) compared to previous IBD trials.
- GEMINI35 (UC) and PURSUIT36 (UC) trials both considered randomised responder populations, and included a mandated steroid taper starting at Week 6. GEMINI reported SFR rates of up to 45.2% following vedolizumab treatment, and PURSUIT reported SFR rates of up to 38.5% following golimumab treatment.
- SERENE-CD37 and SERENE-UC38 trials of adalimumab included steroid tapering from Week 4, during induction, with an SFR rate of 50% at Week 12 (CD), and 39% at Week 52 (in Week 8 responders; UC).
- VARSITY39 (UC) reported SFR rates of 22% with adalimumab and 13% with vedolizumab at Week 52. However, this trial did not include a mandatory tapering protocol, resulting in a more significant cumulative steroid exposure in the vedolizumab group. This makes the results from this study difficult to interpret, reinforcing the importance of a mandatory tapering approach to study design.
In conclusion, corticosteroids are effective at improving symptoms and providing a global sense of wellbeing, but they are ineffective as a maintenance therapy, and toxicity can be significant. This means that SFR is a critical endpoint for evaluating new therapies. Steroid-tapering protocols vary across trials,40 and standardised regimens should be considered in the design of new clinical trials to avoid confusion in the interpretation of results.
Closing Remarks
Doctor Tim Raine
The take-home messages from this symposium are practical steps practitioners can begin taking tomorrow to reduce corticosteroid exposure in their patients.
- Educate patients; if they are offered corticosteroids by their primary care practitioner, they should contact their secondary care team.
- Educate both primary-care and secondary-care colleagues; the role of a secondary-care practitioner is to share knowledge of corticosteroid risks.
- Assess patients for corticosteroid response if treatment is initiated.
- Know one’s own numbers; what fraction of patients are exposed to corticosteroids, and how many have corticosteroid excess? There are simple tools available to gather this information.
- If starting corticosteroid treatment, have an exit strategy in mind.








