Chairperson: Jean-Frederic Colombel1
Speakers: Jean-Frederic Colombel,1 Silvio Danese,2 Laurent Peyrin-Biroulet3
1. Icahn School of Medicine at Mount Sinai, New York City, New York, USA
2. Inflammatory Bowel Diseases Center, Humanitas University, Milan, Italy
3. Nancy University Hospital, Nancy, France
Disclosure: Prof Colombel is a shareholder of Intestinal Biotech Development and GENFIT; has received grant/research support from AbbVie, Janssen, and Takeda; has acted as a consultant or advisory board member for or received honoraria from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly and Company, Enterome, Ferring Pharmaceuticals, Geneva Biotech, Genentech, Gilead, Ipsen, Imedex, Immunic, Janssen, Landos Biopharma, LimmaTech Biologics AG, MedImmune, Merck & Co., Novartis, OMass Therapeutics, Otsuka, Pfizer, Shire, Takeda, TiGenix, and Viela Bio; and has participated in speakers bureau for AbbVie, Amgen, Allergan, Bristol Myers Squibb, Ferring Pharmaceuticals, Shire, and Takeda. Prof Danese has acted as a consultant or advisory board member for or received honoraria from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly and Company, Enthera, Ferring Pharmaceuticals, Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB, and Vifor Pharma. Prof Peyrin-Biroulet has acted as a consultant or advisory board member for or received honoraria from AbbVie, Allergan, Alma S.R.L., Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Boehringer Ingelheim, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly and Company, Enterome, Enthera, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech, Gilead, Hikma, InDex Pharmaceuticals, Inotrem, Janssen, MSD, Mylan, Nestlé, Norgine, Oppilan Pharma, OSE Immunotherapeutics, Roche, Samsung Bioepis, Sandoz, sterna biologicals, Sublimity Therapeutics, Pfizer, Pharmacosmos, Takeda, Theravance, Tillotts, and Vifor Pharma.
Acknowledgements: Medical writing assistance was provided by Stefan Amisten, Amisten Consulting Limited, Epsom, UK.
Support: The symposium and publication of this article were funded by Galapagos NV, Mechelen, Belgium and Gilead Sciences, Inc, Foster City, California, USA.
Citation: EMJ Gastroenterol. 2020;9[1]:30-40.
Meeting Summary
This was a Gilead- and Galapagos-sponsored symposium devoted to today, tomorrow, and the future of ulcerative colitis (UC), as part of the United European Gastroenterology (UEG) Week Virtual 2020.
Prof Colombel welcomed the audience and provided the first talk, summarising the current treatment landscape and unmet needs in UC, and highlighting the limitations of pharmaceutical and surgical therapies for UC, as well as the divergent views of the condition by patients with UC and their physicians.
Prof Danese discussed the late-stage clinical development of sphingosine-1-phosphate (S1P) receptor modulators, IL-23 inhibitors, leukocyte adhesion inhibitors, and JAK/tyrosine kinase 2 (TYK2) inhibitors for the treatment of UC, and considered how these new drugs could change clinical practice.
Finally, Prof Peyrin-Biroulet shared his vision of what the future might hold for the treatment of patients with UC. He summarised the late-stage clinical development pipeline for potential UC therapeutics and shared his view on how the increasing deployment of biosimilars, as well as novel treatment concepts such as dual-targeted biologics and biologic/small-molecule combination therapies, may change the way that UC is treated in the future. He also highlighted the importance of personalised treatment targets and how patient education and patient-specific treatment guidelines could empower patients with UC, to help close the existing gap between routine real-world clinical practice and best practice for the management of UC.
Ulcerative Colitis: Today
Professor Jean-Frederic Colombel
Crohn’s disease (CD) and UC are chronic inflammatory bowel diseases (IBD) that lead to digestive disorders and inflammation in the digestive system.1 Very often, UC is seen as a minor disease; however, UC is a progressive gastrointestinal inflammatory disease of the colon. The extent of colorectal inflammation fluctuates over time and may result in long-term complications, which can be aggravated by structural changes such as strictures, pseudopolyposis, bridging fibrosis, and neoplastic transformation. Functional abnormalities are important because they can cause distressing symptoms, such as urgency and incontinence, and they are linked to decreased contractility and impaired colonic motility. In addition, anorectal dysfunction may lead to ‘lead pipe’ colon, rectal narrowing, widening of the presacral space, and impaired continence.2,3
Systemic, gastrointestinal, and psychological symptoms cause severe disease burden for patients with IBD as they occur with high frequency and severity, and cause significant distress. The top six symptoms include lack of energy, bowel urgency, diarrhoea, flatulence, feeling bloated, and worrying.4 In particular, the lack of energy, experienced as fatigue, and the impact of the psychological burden, such as worrying, were highlighted by Prof Colombel as very important causes of distress in patients with IBD. The prospective, multicountry, observational ICONIC study assessed the cumulative burden in adult patients with UC under routine care, and reported a disconnect between physicians’ and patients’ perceptions, as approximately 40% of patients classified their disease activity differently from their physicians.5 Patients also reported being highly concerned about the disease treatment and potential complications, particularly the potential to require an ostomy bag or to need surgery, unwanted effects of their medication, uncertainty about the course of their disease, and decreased energy levels.6
Although a wide range of therapies are currently approved for the treatment of UC, including anti-IL, anti-integrins, and, more recently, small molecules targeting intracellular processes, (Figure 1),7-10 unmet needs in the treatment of UC remain, both in clinical trials and in clinical practice, with many patients still not able to achieve adequate disease control.11 For anti-TNF drugs, nonresponse rates of 20–40% have been reported in clinical trials and 10–20% in real-world studies.12 Similarly, real-world nonresponse rates of 49–57% have been reported for the α4β7 integrin inhibitor vedolizumab, 38–49% for the IL-12/IL-23 inhibitor ustekinumab in the UNIFI study, and 40–45% for the JAK inhibitor tofacitinib in the OCTAVE study.13,14
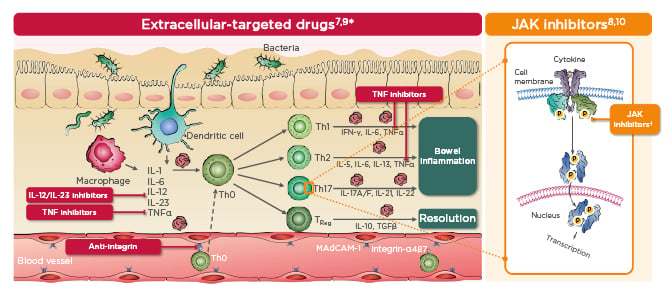
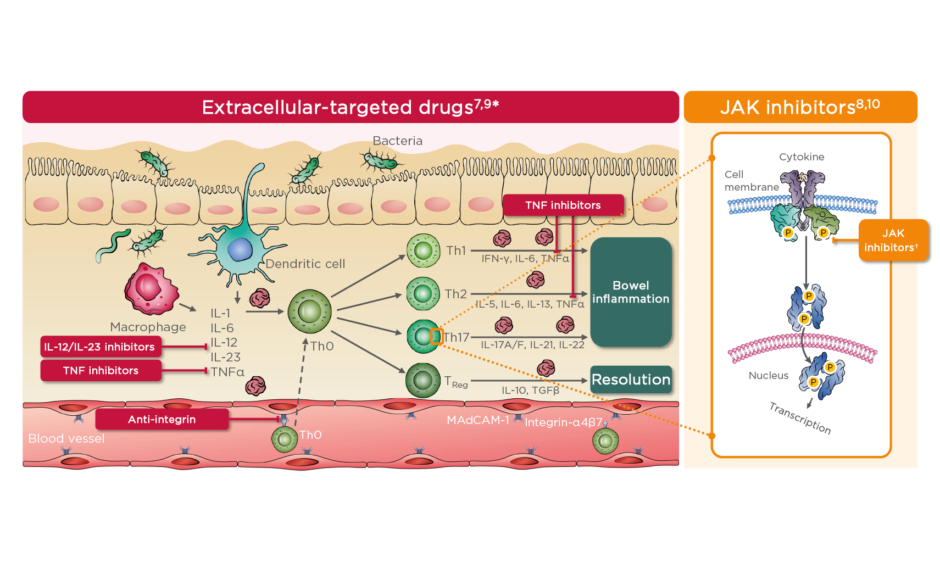
Figure 1: Schematic overview of therapies currently approved for ulcerative colitis.7-10
*Cells and cytokines listed are examples and do not provide an exhaustive list.
†Th17 cells are not the only cells targeted by JAK inhibitors and are used an example to illustrate their action on an intracellular pathway.
MAdCAM-1: mucosal addressin cell adhesion molecule-1; P: phosphate; TReg: regulatory T cell.
Another concern is the plateauing in the rates of steroid-free remission. The proportion of treated patients not achieving steroid-free remission has been reported to be 60–84% with adalimumab or infliximab,15-17 62–87% with vedolizumab in the GEMINI 1 and VARSITY studies,18,19 58–62% for ustekinumab in the UNIFI study,20 and 72% for tofacitinib in the OCTAVE study.14 Although the definitions of steroid-free remission vary between studies, the concept remains useful as a high-hurdle endpoint.
An excessive use of steroids has also been noted in patients diagnosed with IBD and seems to be associated with treatment initiation outside of specialist care, or by a gastroenterologist in training.21 Steroid dependency, defined in the European Crohn’s and Colitis Organisation (ECCO) guidelines as either the prescription of ≥1 steroid over 12 months, the inability to wean from steroids within 3 months, or a disease flare within 3 months of steroid cessation, also remains problematic, particularly in patients with UC.22 An additional burden of steroid therapy is that it may lead to fatigue, which has been reported by nearly 50% of patients with IBD.23
Furthermore, in the real world, not all responders persist on treatment. A real-world study of patients with UC being treated with vedolizumab or infliximab reported that <80% of induction responders persisted for 24 months on treatment.24 Similarly, the Dutch Initiative on Crohn and Colitis (ICC) registry study reported that only 60% of patients who initiated treatment with tofacitinib remained on treatment after 24 weeks.25 Taken together, these illustrate that there is a clear unmet need for new, long-term, effective treatment options for patients with UC.
Colectomy is a major surgical procedure that may significantly affect both mortality rates and the quality of life of patients with UC.26 Although UC colectomy rates have been decreasing since the introduction of biologics,27,28 they still remain high in the long term.29 Furthermore, a majority of patients may not be able to benefit on this decrease in colectomy rates, due to the still limited use of biologics in UC, coupled with an excessive use of steroids.22,30
The research of today shows that UC has a high economic and treatment burden, and patients and physicians do not always share the same view on the disease. There are clear treatment unmet needs, as many patients do not achieve long-term, steroid-free remission without colectomy. Despite updated clinical evidence, new guidelines, and aggressive treatment targets, the early use of effective therapies remains surprisingly uncommon. However, Prof Colombel concluded that the gastroenterological community has nevertheless come a long way: there is now a recognised predictive biomarker (faecal calprotectin) for treatment monitoring, more stringent clinical trial endpoints in the form of long-term remission and histological endpoints, and the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines help to codifying ambitious treatment targets.31-34
Ulcerative Colitis: Tomorrow
Professor Silvio Danese
Emerging therapies for UC currently undergoing Phase III development fall under four main mechanisms of action: leukocyte retention in lymphoid organs by S1P receptor modulators (etrasimod and ozanimod), IL-23 inhibitors (mirikizumab, guselkumab, brazikumab, and risankizumab), JAK/TYK2 inhibitors (filgotinib and upadacitinib), and leukocyte adhesion inhibitors (integrin blockers and etrolizumab).35
S1P modulators are structural analogues of the lipid signalling molecule S1P, with antagonist actions leading to selective immunosuppressive action through the sequestration of lymphocytes in secondary lymphoid tissues and a rapid reduction of peripheral blood lymphocytes.36 In the True North study,37 the S1P modulator ozanimod demonstrated highly statistically significant (p<0.0001) results for the induction of clinical remission at Week 10 and in maintenance at Week 52. Key secondary endpoints of clinical response and endoscopic improvement at Week 10 and at Week 52 were also met, and the safety profile of ozanimod was consistent with that observed in previously reported trials. The leukocyte adhesion inhibitor etrolizumab selectively targets the β7 subunit of both α4β7 and αEβ7 integrins and blocks interactions with their respective ligands, mucosal vascular addressin cell adhesion molecule 1 and E-cadherin, to reduce gut-specific lymphocyte trafficking to the inflamed colon.38 In the HICKORY study, etrolizumab met its primary endpoint of inducing remission versus placebo for patients with UC but failed to meet its primary endpoint versus placebo as maintenance therapy.38 Additionally, in people who had received prior anti-TNF treatment, etrolizumab met the primary endpoint at induction but not at maintenance.39
JAK inhibitors are orally administered small molecules that, by temporarily blocking signalling through the JAK/signal transducer and activator of transcription pathway, inhibit key mechanisms of the innate and adaptive immune response.40 They differ in their selectivity for different JAK: tofacitinib is more selective for JAK1/2/3 than for TYK2, upadacitinib is more selective for JAK1/3 than for JAK2, and filgotinib is more selective for JAK1 than for JAK2.41-43 The blocking of specific JAK kinases by selective JAK inhibitors may be of clinical relevance, as it may translate into therapies with improved safety and efficacy.10
Tofacitinib is the only JAK inhibitor approved for the treatment of moderate-to-severe UC but has shown no efficacy in CD, which has been speculated to be at least partly because of the design of the Phase II and III studies of tofacitinib in CD.42,44,45 Infections and infestations, including a herpes zoster virus safety signal, have been reported for tofacitinib in the OCTAVE Induction 1 and Induction 2 studies,14,46 and a similar safety profile was recently reported in the real-world TROPIC consortium study of 260 patients with UC.47
Preferential JAK1/3 inhibitors, such as upadacitinib, are currently in clinical development for UC.35 Upadacitinib was evaluated in the Phase II part of the U-ACHIEVE study and found during the induction phase to have a safety profile similar to that of placebo; the study is currently recruiting for Phase III.48
Filgotinib, a preferential JAK1 inhibitor, has been evaluated for UC in the combined Phase IIb/III study SELECTION.49 The primary objective of the Phase III part of SELECTION was to evaluate the safety and efficacy of filgotinib in the induction and maintenance treatment of moderately to severely active UC in participants who were either biologic-naïve (n=659) or biologic-experienced (n=689).49,50 The primary endpoints were remission based on components of the Mayo Clinic Score at Weeks 10 and 58, and the use of steroids was tapered during the maintenance phase of the study.49,50 Filgotinib 200 mg demonstrated superior clinical remission in both the biologic-naïve and the biologic-experienced treatment arms, with more patients achieving clinical remission with filgotinib than with placebo during the induction and maintenance phases (11% and 26%, respectively; Figure 2).49,50
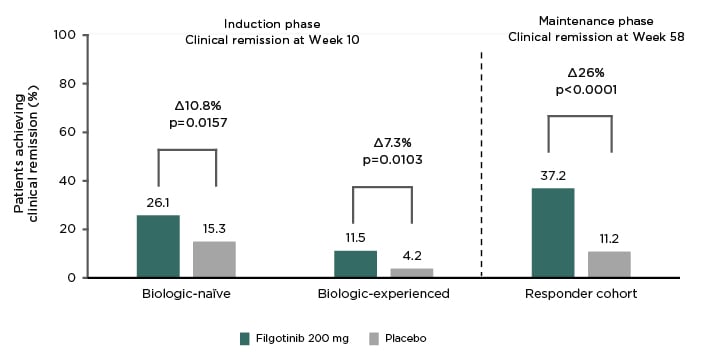
Figure 2: SELECTION primary endpoint results at induction and maintenance.
Figure portrays the proportion of biologic-naïve or biologic-experienced patients with ulcerative colitis achieving clinical remission during the induction phase (Week 10) and for the rerandomised responder cohort during the maintenance phase (Week 58) in SELECTION.
Reproduced with permission from Peyrin-Biroulet49 and Feagan.50
Although adverse events such as infections and infestations were more prevalent in patients treated with filgotinib compared with patients that received placebo during the induction period, rates of herpes zoster were in line with placebo during the maintenance phase (Week 58)50 and were also consistent with the rates observed for patients with rheumatoid arthritis, including those treated with adalimumab or methotrexate.51 Venous thromboembolism rates in SELECTION were also consistent with the low rates observed in rheumatoid arthritis.49,51 Additionally, in the Phase II DARWIN 2 study, filgotinib 200 mg increased the mean levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), and total cholesterol. The LDL:HDL ratio, however, fell slightly over the study period, which indicated that the JAK1 selectivity of filgotinib might lead to proportionally greater increases in HDL cholesterol compared with the increases seen for LDL cholesterol.52
In animal studies, histologic changes have been observed in the testis and in the epididymides at filgotinib doses several-fold higher than the dose recommended for human use. No testicular toxicity has been observed with doses equivalent to the 200 mg dose.53 However, as a precaution and follow-up to this potential safety signal, the randomised, placebo-controlled Phase II studies MANTA (in males with UC or CD) and MANTA-RAy (in males with rheumatic diseases) have been initiated to evaluate the testicular safety of filgotinib in humans.54,55
For adults with moderately to severely active UC, investigational new treatments could, once approved, fit into existing treatment algorithms during both the induction and maintenance phases, with the goal of maintaining steroid-free, clinically and endoscopically defined remission in patients diagnosed with UC (Figure 3).22 Several of the new investigational therapies, such as ozanimod and filgotinib, appear to have benign safety profiles and may, from a safety point of view, be suitable as both first- and second-line therapies (i.e., both before and after biologics). Regarding efficacy, these new molecules could be considered as both first- and second-line therapies, including for long-term maintenance therapy.22
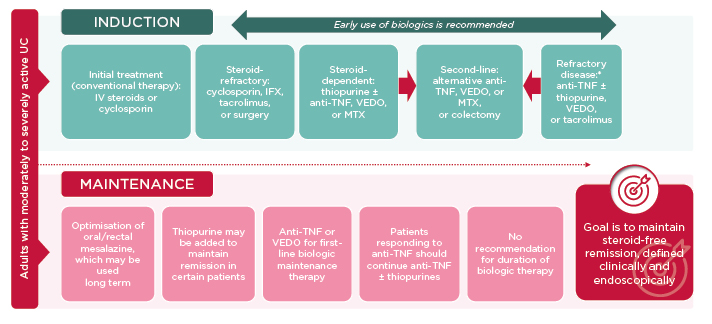
Figure 3: Potential roles of new therapies for the treatment of adults with moderately to severely active ulcerative colitis.
*Refractory to oral steroids or immunomodulator therapy.
IFX: infliximab; IV: intravenous; MTX: methotrexate; UC: ulcerative colitis; VEDO: vedolizumab.
However, patient preferences for oral, intravenous, or subcutaneous modes of administration need to be considered, as modes of administration are becoming more important considerations for novel therapies, particularly as the efficacy and safety profiles of available drugs are plateauing.56 Drug preferences are also influenced by cost, reflected in the higher uptake of biosimilars in Europe compared with the USA, which are more affordable compared with their reference biologics.57 In summary, multiple new therapeutic modalities are in clinical development for UC, and several of these new molecules have shown favourable benefit–risk profiles in late-stage clinical trials.
Ulcerative Colitis: the Future
Professor Laurent Peyrin-Biroulet
Biologics used to treat immunologic conditions such as UC are large molecular weight (>1 kilodaltons [kDa]) protein therapeutics requiring parenteral administration, which preferentially interact with extracellular drug targets. Small molecule therapeutics, on the other hand, are small molecules (<1 kDa) of synthetic origin designed to modulate either intra- or extracellular targets, and are most commonly administered either orally or topically.58,59 Although low trough concentrations of both biologics and small molecule drugs may result in breakthrough symptoms, only biologics are known to be potentially immunogenic, which may lead to the neutralisation of the therapeutic effect of the biologic.60
Biosimilars are defined as biologics with no clinically meaningful differences in efficacy or safety from their licensed originators. The lower cost of biosimilars stimulates market competition and facilitates patient access to biologics because of their lower costs.61 Biosimilars have significantly reduced the treatment cost for biologics both in the European Union (EU) and in the USA, exemplified by the approval of several biosimilar anti-TNF therapeutics for the treatment of immune disorders such as IBD.62-65
Several head-to-head trials are expected to provide some answers regarding the drugs that will constitute the future of IBD therapy. The outcomes of a study,66 which compared the adalimumab biosimilar candidate BI 695501 with EU-approved Humira® (Boehringer Ingelheim, Ingelheim am Rhein, Germany), have recently been reported,67 and results are expected in 2020 from the GARDENIA (etrolizumab versus infliximab) and HIBISCUS (etrolizumab versus adalimumab) Phase III studies.68 In 2021, readouts are expected from the Phase II EXPEDITION trial, which evaluates brazikumab versus vedolizumab in UC,69 and from the Phase III SEAVUE trial comparing adalimumab with ustekinumab in UC.70 Results are also expected in 2022–2023 from the Phase II/III study INTREPID,71 which compares brazikumab with adalimumab, and from the Phase III study VIVID-1,72 which evaluates mirikizumab versus ustekinumab. Outcomes of the Phase II/III study GALAXI,73 which investigates guselkumab versus ustekinumab in patients with moderately to severely active CD, are expected in 2024. In addition, studies evaluating combinations of two different biologics for achievement of remission are being conducted; one example is the Phase II VEGA study, evaluating combination treatments of guselkumab and golimumab in patients with UC.74
“Small molecules have a number of advantages in the treatment of UC” – Prof Silvio Danese, 2020
Although biologics have revolutionised the management of autoimmune diseases,75 small molecules have a number of advantages for the treatment of UC. This is mainly because of their oral mode of administration, effectiveness in patients previously treated with TNF inhibitors, short serum half-life, potential high cost–effectiveness ratio, lack of immunogenicity, previous treatment experiences from other patient types and in other diseases, potential as first-line therapy after aminosalicylates and steroids, rapid absorption time, and potential for use in mild, moderate, and severe UC.76 Prof Peyrin-Biroulet indicated that the lack of immunogenicity associated with small molecule drugs also opens up the possibility of treatment holidays for patients with IBD on small molecule therapies; however, additional research is needed to define which patients and disease stages are most suited for stop/start treatment regimens in IBD.
The immunosuppressive mechanisms of action of drugs used to treat IBD result in reductions in disease activity; however, they are also associated with an increased risk of infections and a potential increase in the risk of developing cancers.77,78 The I-CARE study, a European-wide, prospective, longitudinal, observational, multicentre cohort study, has been designed to evaluate the risk of developing cancer or serious infection in patients that are using immunosuppressive and biologic therapies.79 I-CARE has thus far enrolled >10,000 patients with IBD, and the first results are expected to be presented at ECCO 2021.80
Dual-targeted, or bispecific, antibodies have been proposed as a novel therapeutic approach for the treatment of immune disorders such as IBD. This unique class of biologics combines two distinct binding specificities within a single therapeutic entity, which allows for the simultaneous targeting of two different disease-causing cytokines or pathways by the same therapeutic. Several bispecific biologics are currently in preclinical or clinical development for the treatment of a variety of autoimmune and inflammatory diseases.81
Another approach is combination therapy, in which separate biologics and small molecule drugs are administered concomitantly.82-84 The selection of drugs that might be suitable for combination therapies needs to be based not only on safety profiles, but also on mechanisms of action. However, challenges remain in predicting how effects of crosstalk and synergy from the combination of two different drugs will influence the overall safety and efficacy of a combination therapy. Filgotinib, for example, appears to have a favourable safety profile so could be considered for combination therapy trials with other drugs with favourable safety profiles, such as vedolizumab, ozanimod, or etrolizumab.
Additionally, experimental treatment concepts to modulate immune dysregulation conditions such as IBD are currently being explored, including targeting the human genome, where approximately 99% of the DNA sequence has unknown function, and the gut microbiome.85,86
The future of UC management is envisioned as a stepwise approach, starting with symptom remission (patient-reported outcomes Stages 1–2), followed by endoscopic, histologic, and ultimately molecular healing.87
“The good physician treats the disease; the great physician treats the patient who has the disease” – Sir William Osler, 1903
To achieve this, STRIDE guidelines have been published that include evidence- and consensus-based recommendations for selecting the goals for treat-to-target strategies in patients with IBD. The treatment of patients with UC should target the resolution of rectal bleeding and the normalisation of bowel habits, and outcomes should be assessed every 3 months until symptom resolution and every 6–12 months thereafter. Similarly, the treatment of intestinal inflammation should aim for a Mayo endoscopic subscore of 0 as the optimal target, with a Mayo endoscopic subscore of 1 as a minimum target within 3–6 months after the start of therapy. Histopathology may be used as a sensitive measure of inflammation but not as a target, and available biomarkers, such as C-reactive protein and faecal calprotectin, should be used as adjunctive measures of inflammation for monitoring in patients with UC.34
These ambitious treatment targets may be supported by the implementation of home-based biomarker monitoring, virtual clinics, and telemedicine, which may lead to increased adherence rates and improved treatment outcomes.88 Interest in, and development of, telemedicine and remote monitoring has been greatly accelerated by the ongoing coronavirus disease (COVID-19) pandemic.89
Prof Peyrin-Biroulet outlined his 5-year perspective on the treatment of UC: this included reduced reliance on endoscopy in UC, except for colorectal cancer screening; patients being able to receive treatment from home; targets based on symptoms, endoscopy, and/or histology; and treatments tailored to individual patients and not to molecular pathways, with the ultimate goal of achieving a normal life with UC. Unfortunately, there is still a significant gap between current healthcare practice and optimal treatment strategies in UC90 and more education needs to be aimed at patients rather than at healthcare providers, for example through the development of patient guidelines to educate patients on which treatment options are available.91 The focus also needs to shift from inflammation toward disease modification and quality of life improvements, with the ultimate aim of improving overall patient outcomes.
Conclusion
Although therapies such as biologics and novel small molecule drugs targeting different immune pathways have revolutionised the treatment of autoimmune disorders including IBD in recent decades, unmet needs remain. A new generation of drugs for IBD are in clinical development, including S1P modulators, IL-23 inhibitors, leukocyte adhesion inhibitors, and preferential JAK1 inhibitors. Novel treatment concepts such as bispecific biologics and biologic/small molecule drug combination therapies are also being developed. In parallel, the clinical management of IBD is being improved through the implementation of personalised treat-to-target strategies, biomarker-based disease activity monitoring, and empowerment of patients through improved patient education.