Key Messages
- BeGraft PLUS, a novel covered balloon expandable stent is the latest member of the BeGraft family that complements but does not replace the regular BeGraft peripheral.
- Indicated for restoring and improving the patency and treatment of aneurysms, acute perforations and ruptures of the iliac and renal arteries.
- Suitable for complex lesions and interventions.
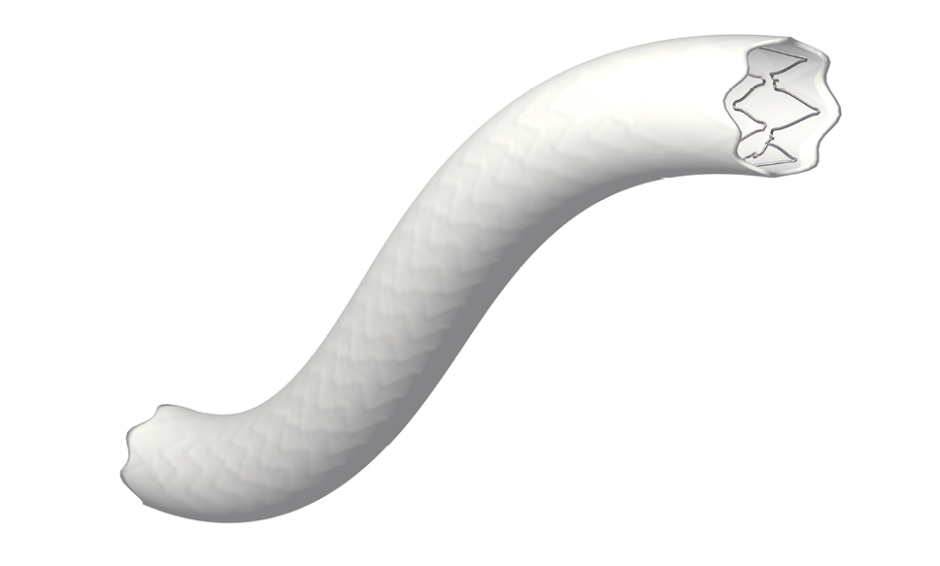
- Has a novel sandwich layer design comprising an outer and inner stent with an outer layer and an extra intermediate layer of ePTFE tubing.
- Official launch presentation for European markets at Leipzig Interventional Course (LINC) 2018, on Wednesday the 31st of January by Professor Eric Verhoeven.
Hechingen, Germany, 31 January, 2018 – Bentley, today, launches its novel BeGraft PLUS, a covered balloon expandable stent, that is the newest member of the BeGraft family, The BeGraft PLUS stent system is approved for intraluminal chronic placement in iliac and renal arteries to restore and improve the patency and treatment of aneurysms, acute perforations, and acute ruptures and fistulas. This indication is the same as that for the BeGraft peripheral stent system, however, BeGraft PLUS has exclusive stent properties that make it more suitable for complex and demanding endovascular lesions and/or vascular defects – notably its high radial force (the highest on the market*) and remarkable flexibility. These features are associated with reliable sealing properties.
“With BeGraft PLUS we see a new generation of covered stents that offer us new options for the treatment of aneurysm disease,” said Eric Verhoeven, MD, PhD, from the Department of Vascular and Endovascular Surgery at the General Hospital Nuremberg and Paracelsus Medical University, Nuremberg, Germany. Professor Verhoeven was a member of the pre-launch group for BeGraft PLUS.
Vascular surgeon, Professor Stéphan Haulon, from Hôpital Marie Lannelongue, Paris, France, is another early adopter of this product. “Recently, we have used a lot of different stents in our daily work, but the BeGraft PLUS is really a different story because it has the highest radial force we have seen so far for covered stents yet remains very flexible, which makes it easy to use in the most complex procedures and complicated lesions,” he remarked.
Novel Wall Construction
One size of stent does not fit all, and explaining a key development with BeGraft PLUS, Professor Verhoeven pointed out that “BeGraft PLUS has a sandwich layer in the wall construction, which makes it more suitable for the treatment of complex aneurysmal disease.” The different unique layers of BeGraft PLUS comprise an outer and inner stent with an outer layer and an intermediate layer of ePTFE tubing, which ensures reliable sealing properties.
Of note, the BeGraft PLUS has up to 3.5 times higher circumferential radial force compared to other balloon expandable covered stents in the market*. “The high radial force ensures a secure connection to the nitinol rings of branched endografts and strong performance in complex lesions,” claims Professor Haulon.
After CE-approval in February 2017, distribution began at selected European centres but the BeGraft PLUS is now available across Europe in lengths ranging 27/28mm, 37/38mm and 57/58mm and including diameters from 5 to 10mm. The BeGraft PLUS is 7Fr. compatible up to a diameter of 8 mm and 8Fr. for diameters of 9 and 10 mm. This is one Fr. larger compared to the regular peripheral BeGraft. BeGraft PLUS does not replace the regular peripheral BeGraft, but is a complementary product.
To date, over 350 patients have been successfully treated with BeGraft PLUS in a small selected group of hospitals.
Notes to the Editors
- Bentley was founded in 2009 by the two medical entrepreneurs Lars Sunnanväder and Miko Obradovic. Bentley quickly grew to a company with over 100 employees and a global distributor network in over 75 countries. Currently, the company has four state-of-the-art products in its portfolio:
- BeGraft coronary, launched in 2012.
- BeGraft peripheral: first generation launched in 2013; second generation, launched in 2015.
- BeSmooth peripheral, launched in 2015.
- BeGraft aortic, launched in 2016.
Bentley has a promising pipeline dedicated to provide new and innovative products to meet the challenges of endovascular surgery and improve clinical outcomes in some of the most complex and demanding procedures.
The company is headquartered in Hechingen, southern Germany.
Media Contact
Bentley Marketing Manager
Martijn Nugteren
Email: [email protected]
Phone: +31 6 188 428 91