Meeting Summary
Obesity has become a serious public health issue worldwide, with its prevalence steadily increasing. The potential consequences of this chronic disease, including cardiovascular disease, increased morbidity, and mortality, pose a significant burden on individuals and healthcare systems. Evidence has established that lifestyle and dietary modification are central to achieving effective weight loss. One approach shown to be efficacious in achieving weight loss is the use of a very low calorie ketogenic diet (VLCKD), which includes stages of induced ketosis, followed by a reintroduction to a low calorie diet and maintenance diet. Such regimens have been shown to result in sustained weight loss and, for some people, remission of Type 2 diabetes (T2D). A similar approach, which may also be a component of the VLCKD, is the use of total or partial replacement of meals using nutritionally complete shakes, bars, or soups. These may be combined with other weight loss measures, including bariatric surgery or medications. It is important that such programmes are delivered in a structured, medically-monitored, and supportive environment, such as laid out by Obesity Canada’s ‘5As’ programme. An ‘obesity shared medical appointment’ model is a multidisciplinary approach, whereby a patient with obesity is seen by a number of healthcare specialists, depending on their comorbidities. The patient also has the opportunity to meet with obesity specialists and engage in monthly patient support groups, all of which have been shown to be successful interventions in helping patients lead a healthier lifestyle, and gain more control over their weight. The following proceedings are based on talks given by leading obesity experts, presented at the 30th European Congress on Obesity (ECO 2023), which took place in Dublin, Republic of Ireland, in May 2023.
Introduction
Obesity is generally defined as having a BMI of ≥30 kg/m2, although, as will be discussed, this measure alone may be insufficient to determine severity of obesity.1 A 2015 survey found that North and Central America leads the world in obesity rates among people ≥15 years of age (25.8–38.2%), followed by Australasia (27.9–30.7%), and several European countries (9.8–30.0%).2 According to a 2016 World Health Organization (WHO) Global Burden of Disease report, more than 1.9 billion adults met the criteria of overweight (BMI ≥25 kg/m2), and roughly 650 million people had obesity.1
Obesity is strongly associated with an increased risk of developing complications, such as cardiovascular disease, cancer, chronic kidney disease, and T2D.3 As such, having a BMI of ≥30 kg/m2 has been associated with premature death. An analysis of the 2017 Global Burden of Disease data found that high BMI accounted for approximately 4.72 million deaths worldwide.4
Studies show that a 5–10% weight loss can lower mortality risk and improve obesity-related comorbidities, including hypertension, dyslipidaemia, polycystic ovary syndrome, gastrointestinal reflux disease, and osteoarthritis.5 Moreover, there is evidence suggesting that weight loss can help delay progression to, or lead to remission of, T2D. In a symposium held at ECO 2023, five leading experts gave presentations focused primarily on the role of weight loss and dietary interventions for people with obesity. Below is a summary of their presentations.
Scientific Update of Overweight and Obesity Treatments and the New International Guidelines of Medical Nutrition
Luca Busetto
According to Luca Busetto, Department of Medicine, University of Padova, Italy, and Center for the Study and the Integrated Management of Obesity, Padova University Hospital, Italy, there is increasing use of a VLCKD for weight management worldwide. This programme, they recounted, “is a powerful intervention that should be carried out under medical supervision.” While the whole programme is called a VLCKD, the ‘active’ stage, which includes a 600–800 kcal/day low carbohydrate, higher fat, and protein diet to induce ketosis is only administered for a few weeks or handful of months. This is followed by a ‘re-introduction’ stage that consists of a 1,200–1,500 kcal/day low calorie diet, and the addition of other foods. At 6 months, the person transitions to the ‘maintenance’ stage, whereby a diet of approximately 1,500–2,200 kcal/day is followed.6
Busetto described how they helped to convene the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO) in 2021, and conducted a meta-analysis of randomised controlled trials to develop guidelines regarding the use of a VLCKD for obesity management.6 They found a significant and meaningful effect of a VLCKD on body weight (-7.06 kg difference; 95% confidence interval: -11.16, -2.97; p=0.0007) and BMI (-2.45 difference; 95% confidence interval: -3.88, -1.01; p=0.0008) at a number of time points over 2 years, compared with other food-based dietary interventions. Loss of fat free mass (e.g., bone and muscles) was similar between people following a VLCKD and those following other diets. The VLCKD also significantly reduced HbA1c and the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index, showing a potential to impact T2D and pre-diabetes.6
The conclusion of the OMTF was the recommendation for use of a VLCKD for individuals with obesity, especially people with obesity-related comorbidities, such as cardiovascular, joint, and/or metabolic diseases. The recommendation also included that such a diet should be personalised, and combined with long-term lifestyle changes that include physical activity and nutritional counselling.6
Busetto concluded their presentation with a discussion on the EASO 2023 position statement on medical nutrition therapy for overweight and obesity in adults, which was developed in collaboration with the European Federation of the Associations of Dietitians (EFAD).7 An update to the adult obesity guidelines was needed, whereby, based on the latest evidence, partial meal replacements were now recommended to help with a reduction in body weight and blood pressure, and improved glycaemic control. Intermittent or continuous calorie restriction may be included in future updates, but the evidence is ‘currently inconclusive.’7
Main Studies on Very Low Calorie Ketogenic Diets in Obesity, and Future Directions
Felipe Casanueva
In this session, Felipe Casanueva, Division of Endocrinology, Department of Medicine, Santiago de Compostela University, Santiago, Spain, discussed how treatments, such as lifestyle changes or hypocaloric diets alone, may not have long-term success in regard to effective weight reduction. While there is some evidence for drug therapy, this may be expensive and, Casanueva emphasised, “we do not know what will happen in 4–5 years when we have not changed the lifestyle of the patient after taking these drugs.” Additionally, they discussed that even though bariatric surgery may be suitable for some, it is irreversible, depending on the type of surgery.5
The concept of protein-sparing to induce ketosis was first introduced in the 1970s by George L. Blackburn and colleagues.8 This has now8,9 evolved to the current VLCKDs, specifically designed to be low in carbohydrates and fats, while maintaining a normal protein content. In a study by Casanueva and colleagues, it was demonstrated that a specific type of VLCKD,10 which is both low in carbohydrates and low in fat content, was advantageous with regard to loss in body weight and visceral fat area over a simple low-calorie diet in the short term (2–6 months), during the ketosis and low-calorie phases, and long term (1–2 years), during the maintenance phase. This diet was also shown to be well-tolerated, without significantly impacting participants’ mood or energy.11 Casanueva also noted in the question and answer session, how another advantage of the specific VLCKD is that in the initial phase “you attain a huge amount of weight loss without hunger.” This, he discussed, means that “patients are motivated by good results to continue.”
Casanueva stressed that a broad approach is needed to treat obesity, which involves assessment of loss in both body fat and muscle mass. This is because sarcopenia, a reduction in muscle mass more typically seen with ageing,12 can be dangerous, as it has been associated with increased morbidity and mortality due to, for example, heart failure via a reduction in left ventricular ejection fraction.13 As such, muscle mass and strength, stated Casanueva, are important factors to monitor in patients undergoing a weight management programme.
With respect to the use of a VLCKD, studies have shown that reduction in free fat mass was predominantly due to total body water loss, most notably extracellular water and not muscle, with a much lower reduction in muscle mass. Studies also observed that changes in measures of muscle strength, despite a mean loss of approximately 20 kg in body weight, did not occur.14 In closing, Casanueva discussed how the VLCKD also led to an increase in anti-inflammatory cytokines and a decrease in pro-inflammatory cytokines, thereby suggesting the potential to reduce the risk of cardiovascular disease and other metabolic diseases.15
Improving Obesity Treatment Engagement and Treatment Response: The Role of Meal Replacements
Jamy Ard
Jamy Ard, Department of Epidemiology and Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA, started their presentation with a discussion on the challenges of asking someone to adhere to a restricted dietary intake.16 Indeed, studies have shown that over time, people tend to decline in their ability or motivation to continue with dietary changes.17 This, Ard noted, is likely due to a number of variables, including degree of nutritional literacy and abilities in the realms of inhibition and restraint. “Meal replacement,” said Ard, “may support patients by dealing with this concept of self-efficacy.” With meal replacements, such as via a complete nutrition shake, soup, or bar, portion control is much simpler. Meal replacement may be partial, where some food-based meals are allowed, or where all nutrition is provided as a total meal replacement (TMR).18 However, Ard pointed out, while the European Union (EU) has detailed guidance regarding energy and nutritional content of meal replacements,19 the U.S. Food and Drug Administration (FDA) does not.
TMR also works through the concept of sensory-specific satiety, such that when a person is presented with a single food stimulus, also termed ‘stimuli-narrowing’, they can soon become satiated by it. However, if presented with a meal that has a high variety of foods, with a large number of food stimuli, such as texture, flavour, and macronutrients, it will likely lead to a higher caloric intake due to lack of sensory-specific satiety.20 This is problematic in the USA, reported Ard, where food portions when eating out tend to be large,16 and/or there is availability of a wide variety of highly palatable food.21 Meal replacements, in contrast, provide few stimulating cues and enhance sensory-specific satiety,22 explained Ard; that is, they provide a meal which, “decreases the hedonic drive for food and increases satiety.” This has been shown via functional MRI studies that highlight how brain regions associated with hedonic drive, self-control, and appetite regulation, such as the dorsolateral prefrontal cortex, exhibit higher executive control, and thus suppress food-associated motivational salience and pleasure when undergoing a period of TMR.23
Ard was involved in the 52-week OPTIWIN trial that evaluated the use of a TMR (800–1,200 kcal/day, dependent on starting BMI) compared with a food-based diet (500–750 kcal/day below estimated total energy expenditure) on weight loss.23 Both study arms also included individual counselling, group behavioural sessions, and moderate to vigorous exercise. In the OPTIWIN study, participants followed a TMR for the first 12–16 weeks, then there was gradual reintroduction of food until Week 26, then 1–2 meal replacements a day, along with food to support weight loss maintenance for the remainder of the study. There were 273 participants who completed the trial, who had an average BMI of 38.8 kg/m2, and were predominantly female (82%) and White (71%).24
Results showed a significantly greater weight reduction (p<0.001) with the TMR diet at both Week 26 (mean: -12.4±0.6%) and Week 52 (mean: -10.5±0.6%) compared with the food-based diet (mean: -6.0±0.6% and -5.5±0.6%, respectively). Also observed in the TMR group was a significantly greater percentage of participants who achieved a weight loss of ≥5%, ≥10%, or ≥15%. For instance, at Week 52, 43.7% of the TMR participants had a ≥10% weight loss compared with 21.7% of those on the food-based diet (p<0.001).25
In the UK DiRECT trial, 157 adults with T2D in a primary care setting followed a TMR (825–853 kcal/day) for 3 months, followed by a structured food reintroduction phase, and finally a maintenance phase for the remainder of the study, all of which included monthly visits to support long-term weight loss maintenance. This was compared with the standard primary care guideline-driven dietary intervention (n=149). At 1 year, average weight loss in the TMR group was 10.0±8.0 kg compared to 1.0±3.7 kg in the control group (p<0.001).25,26 Overall, 46% of the TMR group compared with 4% of the control group achieved remission of diabetes (HbA1c <6.5% after 2 months without diabetes medication), with 86% of the participants who lost ≥15 kg achieving diabetes remission.25 At 2 years, while only 3% of the control group had remission of diabetes, roughly 36% of the TMR group still had remission of their diabetes (p<0.0001).26
To conclude their presentation, Ard discussed the DROPLET trial, whereby 278 adults in primary care were randomised to standard of care or a TMR diet over a shorter period of time (810 kcal/day for 8 weeks, followed by slow transition back to normal food). This study found that 45% of participants in the TMR group had a 10% or more weight loss at 12 months, compared to 15% of participants in the standard care group, despite the TMR only being administered for a limited period of time.27
In summary, Ard discussed how these meal replacement studies highlight the validity of this approach for weight management in patients with obesity.
Analysing the Appropriate Way of Treating Obesity
Bart Van der Schueren
“Most individuals living with obesity,” said Bart Van der Schueren, University Hospitals Leuven, Belgium, and Laboratory of Clinical and Experimental Endocrinology, University of Leuven, Belgium, “have tried multiple times to lose weight.” This may be because patients have reported a lack of useful information and sensitivity from their physicians, and the need for tailored, supported weight management strategies. Moreover, Van der Schueren advised that patients need to be counselled that obesity is a chronic disease, and that management of obesity is not just about weight loss, but also about improving health. It is important, they stressed, that weight management programmes are individualised to meet the patient’s needs, and that the root causes of obesity are addressed and roadblocks removed.
These principles are embodied in Obesity Canada’s 5As of obesity management: ask, assess, advise, agree, and assist (Figure 1).28,29 It is important, Van der Schueren emphasised, that all of the ‘As’ are addressed, as research shows that while many physicians routinely ‘ask’ and ‘advise’ patients about weight loss, few actually ‘assess’ them, ‘agree’ on a plan, or ‘assist’ them with implementing a plan.30
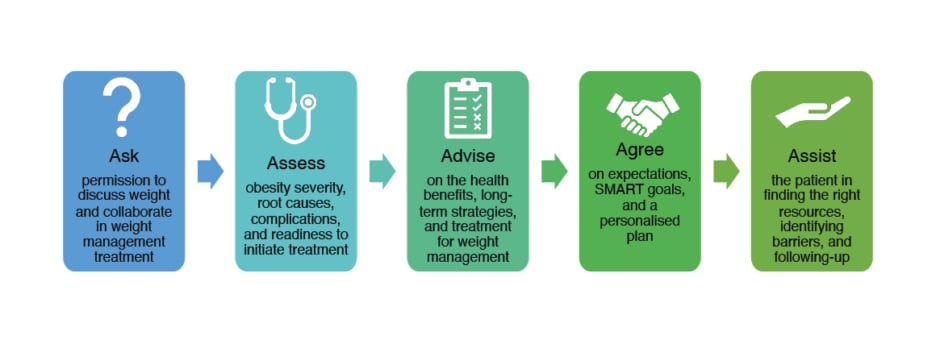
Figure 1: Obesity Canada’s 5As obesity management programme.28,29
SMART: specific, measurable, attainable, realistic, and time-bound.
The first A, asking permission to discuss weight, is important because many people with obesity have had weight-stigmatising experiences, especially during childhood into young adulthood, and do not want to discuss their weight or an intervention.31 As weight stigma can lead to lifelong damaging effects, a multidisciplinary group of international experts convened and developed the joint international consensus statement for ending stigma of obesity.32 “If we really want to take care of patients properly,” said Van der Schueren, “we should throw the stigma away and treat it as any other disease, and not put all responsibility on the patient.”
With regard to the second A, ‘assess’, Van der Schueren discussed how not only physiological measures should be assessed, but also whether or not having obesity presents a risk to the person’s health.28 This is due to a person’s BMI being a relatively poor predictor of survival, as evidenced by a study showing similar survival rates in people who are overweight (25.0–29.9 kg/m2) or with Class III obesity (≥40 kg/m2).33 The Edmonton Obesity Staging System (EOSS)34 ranks people from Stage 0 (no clinical risk factors) to Stage 4 (end-stage disease), considering medical, physical, and psychological parameters, to describe comorbidities associated with excess weight. This means that even if you are in the Class III obesity range by BMI, it is possible to be at an EOSS Stage 0 or Stage 1 (subclinical risk factors).33 This is important, as EOSS stage has been found to be far more predictive of survival rate, decreasing greatly with increasing stage number, compared to BMI.35
The next ‘A’ is ‘advise’. This includes discussing with the patient the risks of obesity and the benefits of even modest weight loss, as well as advising on different treatment options, and the need for a long-term strategy.28,29 In regard to agreeing on goals with the patient,28 Van der Schueren discussed the use of ‘SMART’ goals, meaning they need to be ‘specific’, ‘measurable’, ‘attainable’, ‘realistic’, and ‘time-bound’.29
The final ‘A’ is assisting the patient with identifying resources, barriers, and long-term follow-up, which may also include the use of injectable medications, such as liraglutide or semaglutide.28,36,37 Of note, though, studies have shown that weight loss is typically only during the use of these types of medications, and weight regain will occur after the medication is stopped, even to a weight above a person’s initial starting weight.37 This means that patients need to either be counselled that use of a medication(s) will be a chronic treatment or, if the medications are stopped, further measures will need to be implemented to prevent post-medication weight regain. Additionally, it is of note that not only body fat mass may be lost with these injections, but also lean body mass.36
Van der Schueren concluded by discussing how important it is that patients seek out help early on. They recounted how many may try and tackle obesity themselves and only see an obesity team to discuss surgery, which should not be the case. Thus, conversations should begin at the primary care level to lessen the burden and long wait time to see a specialist.
An Interdisciplinary Approach to Obesity Therapy
Bartolomé Burguera
The prevalence of obesity and severe obesity is rising.1,38 However, this is not a new condition, as Bartolomé Burguera, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Ohio, USA, discussed that there are records of weight management treatments dating back to the 10th century with regimens similar to modern times, including physical activity, dietary intervention, medication, and stress reduction.39,40
Burguera reported that the Cleveland Clinic, Ohio, USA, manages approximately 25,000 patients with obesity a month, around 15,000 of whom have diabetes; however, fewer than 3% of these patients are seen solely to address obesity.41 “So,” Burguera said, “we are not addressing the primary problem.” Burguera and colleagues developed an intensive lifestyle intervention model that includes group sessions to provide advice and discuss dietary habits, daily behaviour changes, and, where appropriate, weight loss medications. Burguera and colleagues conducted the 24-month TRAMOMTANA study to compare three groups: Group 1 (n=60) received this intensive lifestyle intervention, including behavioural therapy and nutritional counselling; Group 2 (n=46) received conventional obesity therapy, including standard medical treatment; and Group 3 (n=37) underwent bariatric surgery, along with an associated programme.42 While the bariatric surgery group showed the most weight loss (29.6%), Burguera discussed that many patients with obesity are not eligible to undergo this procedure, and thus are typically left with the option of standard of care, which showed a mean percentage weight loss of only 1.6% compared with 11.3% in the intensive lifestyle intervention group.42
Findings from this study led to development of the ‘obesity shared medical appointments’ (SMA) programme at the Cleveland Clinic, which involves endocrinologists, obesity specialists, advanced practice providers, dietitians, exercise physiologists, psychologists, pharmacists, and a social worker. Monitoring of patients includes examination of body composition, including fat, bone, and muscle mass. There are also Diabetes (Obesity) Centers that cover departments such as geriatrics, paediatrics, and preventative endocrinology.
Once enrolled in this programme, the patient will initially be seen by one healthcare provider, followed by the specific specialists needed. After 4 months, there will be monthly group visits to the clinic, involving eight−10 patients that meet with a dietitian and an obesity specialist to learn about specific obesity/diet-related topics, and ask questions to address individual concerns. There may also be an exercise component during the group sessions. At the end of 6 months, the group will transition to virtual visits for the maintenance period. Studies prior to and during the COVID-19 pandemic showed there were no significant differences in weight loss between patients who attended the group sessions in-person or virtually, and had similar rates of dropout.43,44 There are currently approximately 1,400 patients at the Cleveland Clinic in this obesity SMAs programme, with studies by Burguera and colleagues confirming that participation in this programme can lead to an improvement in patients’ general health, and more control over their weight.43,45
Burguera commented that “losing weight is not that complicated. The complicated part is maintaining the weight loss.” As such, they stressed the importance of a long-term, multidisciplinary weight management programme to help with sustaining weight loss. This requires a number of tools and resources, such as education on nutrition, personalised exercise programmes, sleep management, addressing anxiety and depression if present, and utilising medications and bariatric surgery if a medical approach is not successful. Studies have shown that patients are able to achieve greater weight loss over an extended period of time with use of an SMA programme compared with patients who do not use an SMA.46 Findings from a clinical trial where participants received a weight management programme consisting of monthly visits with advice and discussion on healthy lifestyle changes alone (n=100), or the same weight management programme but with addition of anti-obesity medications (n=100), showed a significantly higher (p<0.01) mean change from baseline in percentage body weight in the combined group [estimated mean: -7.7%; standard error: 0.7%) versus the weight management programme only group (-4.2%; standard error: 0.7%).47
Burguera concluded that, as current obesity therapy approaches are ineffective in long-term maintenance, there is a need to maximise novel care delivery initiatives utilising interdisciplinary care, SMAs, and digital and technological support, so that people with obesity have a long-term therapeutic plan.
Conclusion
The five leading experts presented dietary interventions that take a more holistic and long-term approach to weight management in people who have obesity. This may include a VLCKD, TMR, or partial meal replacement, sometimes combined with surgery or medication, but always involving components whereby patients are monitored and supported throughout using novel care delivery initiatives.







