BACKGROUND
Myelodysplastic syndromes (MDS) are highly heterogeneous clonal haematopoietic disorders.1 Allogeneic haematopoietic stem cell transplantation (HSCT) remains the only curative treatment for MDS and is of special interest in patients with a high risk of progression to acute myeloid leukaemia (AML).2 It has been shown that, in MDS, CD34+/CD38- cells possess MDS stem cell potential and that clones of secondary AML originate from the MDS disease stage.3,4 However, no study has evaluated the prognostic impact of the MDS stem cell-containing cell population burden in MDS patients prior to therapy.
AIM
The aim of the study was to analyse the prognostic impact of CD34+/CD38- cell burden in MDS patients receiving allogeneic HSCT.
METHODS
We retrospectively analysed 124 patients diagnosed with MDS (n=105) or myelodysplastic/myeloproliferative neoplasm (n=19) receiving HSCT at our institution. The median age at HSCT was 61.3 years (range: 22.2–74.4). The conditioning regimens used were reduced intensity (44%, fludarabine with busulfan or treosulfan) or non-myeloablative (56%, fludarabine with 2Gy or 3Gy total body irradiation). Prior to HSCT, 59% of patients received cytoreductive therapy with hypomethylating agents (25%), AML chemotherapy (24%), or both (10%). Median follow-up after HSCT was 4.3 years. Karyotype analyses were performed centrally at our institution. Risk values according to the Revised International Prognostic Scoring System (IPSS-R) were 7% low, 28% intermediate, 21% high, 36% very high, and 7% unknown. The CD34+/CD38- cell burden was evaluated by flow cytometry in untreated bone marrow material. Using R’s OptimalCutpoint package, a 1% CD34+/CD38- cell cut-off was determined and divided the cohort into patients with a high (34%) or low (66%) CD34+/CD38- cell burden.
RESULTS
A high pretreatment bone marrow CD34+/CD38- cell burden was associated with a higher bone marrow mononuclear cell expression of CD13 (p<0.001), CD33 (p<0.001), and CD117 (p<0.001), an excess of blasts (p<0.001), and worse IPSS-R risk group (p=0.03). Patients with a high CD34+/CD38- cell burden had worse IPSS-R genetic risk (p=0.02); were more likely to have an abnormal (p=0.04), complex (p=0.002), or monosomal (p=0.004) karyotype; and more often received cytoreductive treatment prior to HSCT (p=0.008). Patients with a high CD34+/CD38- cell burden had a significantly higher cumulative incidence of relapse or progression (CIR; p<0.001; Figure 1A), a higher cumulative incidence of secondary AML (CIsAML; p<0.001; Figure 1B), and a shorter overall survival (OS; p=0.12) by trend. In multivariate analyses, a high CD34+/CD38- cell burden remained an independent prognostic factor for higher CIR (hazard ratio: 2.88; p=0.005) after adjustment for IPSS-R risk, for higher CIsAML (hazard ratio: 3.13; p=0.02) after adjustment for IPSS-R risk, age at HSCT and human leukocyte antigen match, and for shorter OS (odds ratio: 0.47; p=0.01) after adjustment for pre-HSCT bone marrow blast count, human leukocyte antigen match, and donor type. Analysing IPSS-R low or intermediate and high or very high risk MDS patients separately, a high CD34+/CD38- cell burden indicated patients with higher CIR (p<0.001), higher CIsAML (p=0.003), and shorter OS (p=0.12) by trend irrespective of the IPSS-R risk group.
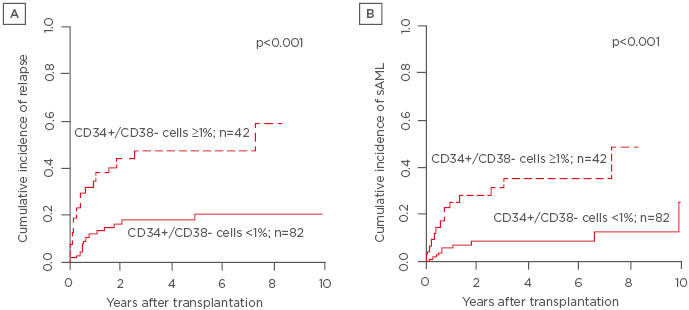
Figure 1: A) Cumulative incidence of relapse and B) cumulative incidence of secondary acute myeloid leukaemia.
sAML: secondary acute myeloid leukaemia.
CONCLUSION
In conclusion, a high pretreatment CD34+/CD38- cell burden was associated with higher CIR, higher CIsAML, and a trend for shorter OS after HSCT. Despite the correlation with high-risk disease, a high CD34+/CD38- cell burden provided independent prognostic information for all endpoints in the multivariable analyses and in separate analyses for IPSS-R low or intermediate and high or very high-risk patients. The observed prognostic impact is likely mediated by MDS stem cells within the CD34+/CD38- cell population, initiating MDS relapse or progression to AML.







