BACKGROUND AND AIMS
Fanconi anaemia (FA) is a rare, inherited bone marrow failure syndrome, for which the only available curative treatment is haematopoietic cell transplant (HCT).1 Gene therapy has been reported to improve bone marrow function in individuals with FA. However, the clinical application of gene therapy is still in its initial stages with controversy over whether gene therapy can potentially replace HCT.2 This systematic review compares the long-term safety and clinical outcomes of gene therapy versus the standard treatment of choice, i.e., HCT, in FA patients, using the peer-reviewed literature published on this topic.
MATERIALS AND METHODS
This systematic review followed the PRISMA Guidelines. The primary objective was to determine the symptom-free survival rate, serious adverse events, and long-term survival probability of individuals with FA, attributable to gene therapy. Secondary objectives included assessing post-infusion blood cell counts, the use of blood transfusions, or the need for a bone marrow transplant and quality of life post-gene therapy. Randomised controlled trials, prospective clinical trials, retrospective studies, case reports, and case-control studies were included. FA patients, of all ages and gender, irrespective of geographical or healthcare setting, were considered. Studies (published between 2005 and 2019) focussing on gene therapy with or without standard treatment were included. Table 1 details the studies that were included in the final study.
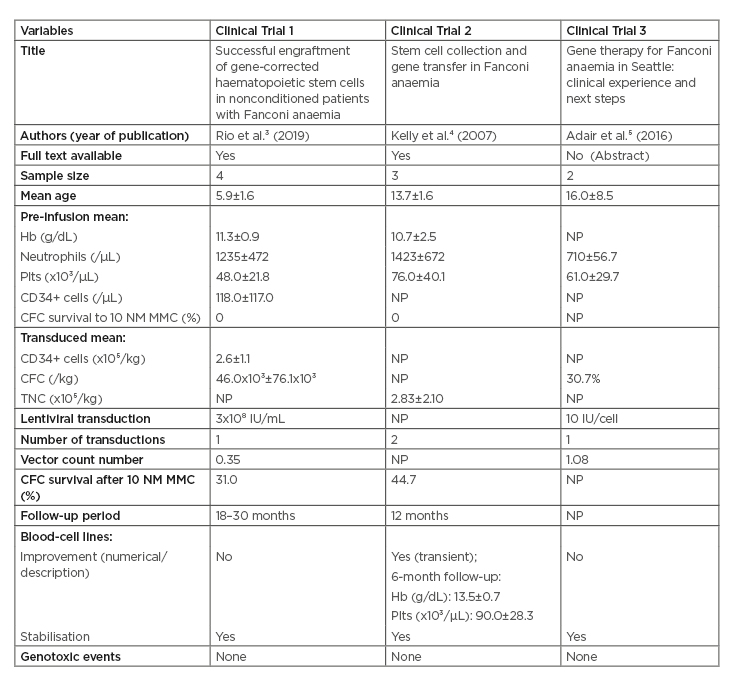
Table 1: Summary of the clinical trials included.
CFC: colony-forming cells; Hb: haemoglobin; MMC: mitomycin C; NM: nonmelanoma; NP: not provided; Plts: platelets; TNC: total nucleated cells.
The authors conducted electronic searches of PubMed, Web of Science, Cochrane Library, and Google Scholar, and screened the reference lists of the included studies to identify potentially relevant studies. The full texts of articles that met the inclusion criteria were reviewed. Reviewers independently extracted data from the included studies using a designed structured data form. The authors assessed the risk of bias using the Cochrane Risk of Bias tool for randomised controlled trials, and also planned to calculate whether statistical heterogeneity was present using the chi-square test for homogeneity, with p<0.1 as significant (anticipating a very small sample size). Assessment for publication bias was planned if at least 10 studies were to be included in the final selection.
RESULTS
Three clinical trials were included in the final sample applying strict selection criteria.3-5 A total of nine patients were therefore included in the study, with a mean age of 10.7±5.7. The mean pre-infusion haemoglobin (g/dl), neutrophils (/µL), and platelets (103/µl) were 11.0±1.6 (two studies), 1,181±525, and 61.1±28.7, respectively, and an overall vector count number (VCN) of 0.59±0.60 (two studies). All patients had lentiviral-mediated gene therapy. A 1-year follow-up of most patients showed stabilisation and mild improvement in blood lineages, without any serious genotoxic events. There were no cytogenetic abnormalities reported in patients in two of the studies. A meta-regression analysis could not be conducted due to the absence of randomised trials and limited availability of patient data. Overall, very little long-term follow-up data in any of the patients was observed, thus the study objective of comparing gene therapy to HCT remains inconclusive and unanswered. The authors were also unable to assess quality of life after gene therapy since this was not reported in any study.
CONCLUSION
Gene therapy is an overall safe procedure for FA; however randomised studies need to be conducted to gain a better insight into the beneficial long-term effects it has in patients with FA, and if there is potential to become a standard-of-care treatment.







