BACKGROUND
Ferumoxytol is an intravenous (IV) formulation of iron that can be infused quickly, making it a convenient choice. It is typically given in 2 doses of 510 mg. Auerbach et al.1 previously described utilising a single dose of 1,020 mg over 15 minutes safely and effectively. A total dose infusion of 1,020 mg has been described by experts in the field as the “maximum safe dose.”2,3 There exists very little published literature about the safety and efficacy of this off-label dosing; however, it is an attractive administration schedule due to convenience of the one-time dose. In July 2018, the authors began to administer a single 1,020 mg dose of ferumoxytol to patients diagnosed with iron deficiency at the north Florida/south Georgia Veterans Health System in Gainesville, Florida, USA. The purpose of this review is to evaluate the impact of the use of the 1,020 mg ferumoxytol dose to ensure safe, effective, and efficient utilisation in the management of iron deficiency anaemia.
METHODS AND RESULTS
A retrospective chart review was conducted on patients who received ferumoxytol from February 1st 2018 to January 31st 2019 to capture approximately 6 months of data prior to, and after, the dosing strategy change. Patients were excluded from review if they had received IV iron within 3 months prior to the study period. Parameters collected included pre and post haemoglobin, iron saturation and ferritin concentrations, dose of iron, frequency and number of infusions, post infusion monitoring time, and hypersensitivity reactions. The primary outcome was assessing safety, particularly the rate of infusion reactions for the entire cohort of patients. Secondary outcomes included efficacy and clinic utilisation. Number of visits, baseline and change in haemoglobin, ferritin and iron saturation following 1 dose of 1,020 mg, or 2 doses of 510 mg were compared using paired t-tests. Rate of infusion reactions was compared between all patients who received either dose using Fisher’s exact test.
A total of 212 patients were screened and 140 included in the analysis. During the study period, 270 total doses of iron were given. Fifty-nine patients (42%) received only 510 mg doses and 60 (43%) received only 1,020mg doses and were included in the efficacy analysis. An additional 21 (15%) received both 510 mg and 1,020 mg doses and were included in the analysis of reaction rate. Baseline characteristics were similar between the groups (Table 1). Response to iron infusions were not significantly different between the dosing strategies. Mean change in haemoglobin was 1.96 g/dL for the 510 mg group and 2.00 g/dL for the 1,020 mg group (p=0.726). Mean change in ferritin was 114 ng/mL and 120 ng/mL (p=0.8203). Likewise, mean change in iron saturation was 13.6% and 14.3% (p=0.7808). The rate of infusion reactions was not increased with the higher dose, with only 1 reaction occurring in each group (0.57% and 1.04%; p=1.00). Both infusion reactions were able to be treated on an outpatient basis and the patients were discharged from the infusion clinic on the same day. Administering the 1,020 mg dose significantly reduced the number of infusion room visits required, with an average of 2 visits for 510 mg patients and 1 visit for 1,020 mg patients (p<0.0001).
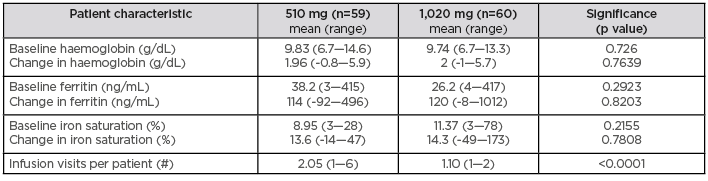
Table 1: Comparison of patients treated with ferumoxytol 510mg and ferumoxytol 1,020 mg.
CONCLUSION
In conclusion, implementation of a total dose infusion of 1,020 mg of ferumoxytol reduced the number of infusion room visits without increasing infusion reactions or compromising efficacy. This strategy could be considered at other institutions to improve infusion room access, patient convenience, and reduce costs.







