Abstract
Acute myeloid leukaemia (AML) is a highly heterogeneous disorder and is characterised by the proliferation of poorly differentiated myeloid cells due to underlying mutation, eventually causing bone marrow failure. Accounting for approximately 25% of cases, AML is the most frequent form of leukaemia in the world yet has the lowest survival rate among all leukaemias. Patients with haematological malignancy are more susceptible to severe acute respiratory syndrome coronavirus-2 infection and further development of severe infection, including pneumonia with poor blood oxygenation. The management of such patients is more challenging than expected. Successful management of one such case is discussed in this report. COVID-19 infection can cause great harm to a patient with underlying leukaemia and increase the mortality risk. It has a major impact on the physical and psychological health of the patient. Therefore, these patients need special care and attention. The authors emphasise the importance of supportive management (oxygen with bilevel positive airway pressure, prone positioning, and physiotherapy) to prevent complications.
INTRODUCTION
The COVID-19 outbreak, first identified in the Wuhan district of China in December 2019, was declared a pandemic by the World Health Organization (WHO) in March 2020.1 The virus predominantly targets the respiratory system, causing symptoms of varying severity, with acute respiratory distress syndrome (ARDS) representing the most severe form and requiring intensive care. Patients who are of an advanced age or with comorbidities such as diabetes, heart disease, and an immunocompromised state are at the highest risk of death.2 Patients with cancer constitute a significant caseload of the diseased population. The current pandemic has further increased the susceptibility of such patients to infections.3 One such risk group of patients who have a high mortality rate with COVID-19 infection are patients with haematological malignancies.4 However, patients with benign disorders like sickle cell disease are also prone.5
Acute myeloid leukaemia (AML), which makes up 80% of leukaemias in the adult population, is a highly heterogeneous disorder characterised by the proliferation of poorly differentiated myeloid cells due to an underlying mutation, eventually resulting in bone marrow failure.6 Incidence of AML increases with age, with the majority occurring in those over 65 years. Despite the improvement in outcomes with recent advances in treatment, the prognosis in older patients is still lacking.7 This article hereby reports a case of a patient with known AML who presented with a severe COVID-19 infection. There is a paucity of literature regarding the management of severe COVID-19 infection in a known case of AML malignancy, and hence the authors found it worthwhile to share the successful management of one such case with a review of the literature.
CASE
A 45-year-old male patient was diagnosed with AML in January 2020 in a tertiary care hospital where he was admitted for drainage of perianal abscess. The patient was of average build, recently diagnosed with Type 2 diabetes mellitus, and had no other associated illness. His initial workup revealed pancytopenia, with atypical white blood cells in the peripheral smear. Bone marrow aspiration and biopsy showed the presence of 20% blast cells. Further morphological and flow cytometric analysis of the bone marrow specimen was completed, and the patient was diagnosed with AML-M4. Treatment was induced with cytarabine and daunorubicin; in total, three cycles of chemotherapy were given within 6 months, with the last cycle administered in June 2020. After the second cycle of chemotherapy, the patient developed febrile neutropenia and was managed with intravenous antibiotics and granulocyte colony-stimulating factor.
Six months later, during the COVID-19 pandemic, he developed a mild-to-moderate fever on 20th June 2020, with a dry cough and shortness of breath. He denied any history of chest pain, anosmia, and diarrhoea. He consulted elsewhere for this complaint, where he was diagnosed with COVID-19 before being referred to his tertiary care institute.
At presentation in the authors’ emergency department, he had progressive difficulty in breathing on 24th June 2020, with a respiratory rate of 34 breaths/min and oxygen saturation (SpO2) of 94% on 12 L oxygen through a non-rebreathing mask (NRBM). Other vital parameters included a heart rate of 102 beats/min, non-invasive blood pressure of 130/90 mmHg, and an axillary temperature of 98.5 °F. A real-time PCR (RT-PCR) on his nasopharyngeal swab repeated at the authors’ institute confirmed COVID-19. His chest X-ray revealed bilateral lung infiltrates with a patch of consolidation in the right lower zone.
The patient was shifted to the COVID-19 intensive care unit for further management. A high-resolution CT (HRCT) chest done in the first week of admission (on 27th June 2020) revealed extensive bilateral ground-glass opacities with sub-pleural, inter-lobular septal thickening, which is consistent with a diagnosis of COVID-19 (Figure 1). The laboratory tests revealed an elevated white blood cell count and C-reactive protein.
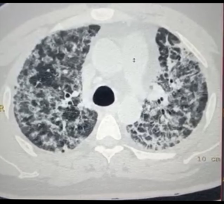
Figure 1: High-resolution CT of the patient’s thorax on Day 4.
All investigations performed over a period in the intensive care unit are summarised in (Table 1).
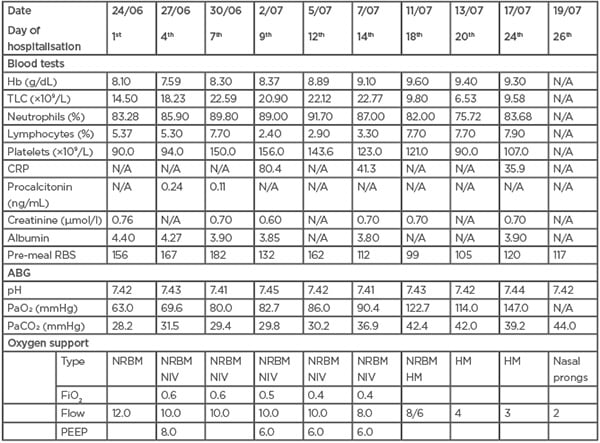
Table 1: Routine investigations of a patient with diagnosed acute myeloid leukaemia who tested positive for COVID-19 in the intensive care unit.
ABG: arterial blood gas; CRP: C-reactive protein; FiO2: fraction of inspired oxygen; Hb: haemoglobin; HM: Hudson mask; N/A: not applicable; NIV: non-invasive ventilation; NRBM: non-rebreathing mask; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; PEEP; positive end-expiratory pressure; RBS: random blood glucose; TLC: total leukocyte count.
The patient met the criteria of severe ARDS with a partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio of <100. He was kept on a NRBM initially at 12 L/min. However, due to persistent hypoxia (SpO2: 85–90%), non-invasive ventilation (NIV) was applied intermittently, with continuous positive airway pressure and settings of FiO2 of 60%, positive end-expiratory pressure of 8 cmH20, and pressure support of 6 cmH2O for 2 hours. The patient was advised to maintain a prone position for at least 10–12 hours daily, with regular chest physiotherapy with an incentive spirometer. He was also encouraged to change his position every 2 hours to left lateral or right lateral if he found the prone positioning challenging to maintain for prolonged hours.
Simultaneously, he was started on parenteral antibiotics (meropenem 1 gm three times a day and vancomycin 1 gm twice a day) and parenteral antifungal (voriconazole 200 mg twice a day). In addition, 6 mg of dexamethasone was given once daily as per the RECOVERY trial. Supportive treatment in the form of intravenous fluids, paracetamol, and pantoprazole was instituted.
Thrombo-prophylaxis was ensured mechanically with an intermittent pneumatic compression device, and pharmacologically with low-molecular-weight heparin, ensuring adequate platelet counts. The patient was kept nil per oral initially for a couple of days, while contemplating high chances of endotracheal intubation if required, and was gradually shifted to an oral liquid diet. The patient’s high blood glucose levels (pre-meal random blood glucose: 140–200 mg/dL) were managed with insulin therapy.
NIV settings were revised to FiO2 of 40% on the ninth day, positive end-expiratory pressure to 6 cmH2O, and pressure support to 5 cmH2O. After the second week, there was a significant clinical and radiological improvement, with reduction in oxygen requirement, maintaining adequate oxygen saturation on an alternate Hudson mask (4–6 L/min) and non-rebreathing mask (10 L/min) for 2 hours and 4 hours, respectively.
By the third week, he maintained a good SpO2 on a Hudson mask alone, and PaO2 levels in arterial blood gas had also improved. However, another chest HRCT in the third week (13th July 2020) suggested severe disease, with a CT severity score of 37/40 and features of early fibrosis (Figure 2).
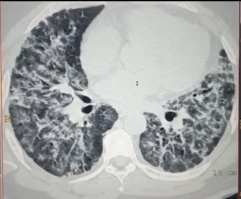
Figure 2: High-resolution CT of the patient’s thorax on Day 19.
In the third week (9th July 2020), antibiotics were de-escalated to piperacillin and tazobactam injection, which was stopped after 7 days as he remained afebrile with decreasing white blood cell counts and a sterile blood culture report. IL-6 levels were within the normal range (18.4 pg/mL; 20.4 pg/mL).
In the fourth week, he was shifted to a high dependency unit on nasal prongs at 2–4 L/min on account of improved blood oxygenation levels and no new findings in his chest X-ray.
Throughout his hospital stay, COVID-19 nasopharyngeal swab RT-PCR testing was repeated, weekly; once for the initial 3 weeks and then every fourth day as per the hospital protocol. After 1 month, his COVID-19 RT-PCR report came negative, and he was discharged with a home isolation protocol and advised follow up after 2 weeks.
REVIEW OF LITERATURE AND DISCUSSION
Search Strategy
The paucity of literature on patients with leukaemia infected with COVID-19 prompted the authors to highlight one such rare case, admitted and managed at their institute with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The search was limited to human studies published in the English language in PubMed, Embase, Google Scholar, and Cochrane, from 1995 to February 2021.
Bibliographies and references of selected publications on critical care management of patients positive for COVID-19 diagnosed with AML were manually screened. The full text of each article was studied once the abstract was analysed by the reviewer and being found appropriate. The decision to include a study in the final analysis was based on an independent assessment performed by another reviewer. The authors conducted the literature search themselves. The initial electronic search using medical subject headings (such as “critical care,” “acute myeloid leukemia,” “AML,” “COVID-19,” and “Coronavirus”) and PubMed searches were emphasised the most.
Acute Myeloid Leukaemia
Definition and epidemiology
Leukaemia comprises <3% of all cancers and is the leading cause of death in children and young adults. AML is defined as cancer involving blood and bone marrow, arising from clonal expansion of malignant haematopoietic precursor cells, which, if left untreated, can be lethal. It is also known as acute myelogenous, myeloblastic, granulocytic, or non-lymphocytic leukaemia. Accounting for approximately 25% of leukaemias, AML is the most frequent form of leukaemia globally, with the lowest survival rate. It has a slight male predominance in adults in most countries.8
Pathogenesis and risk factors
In AML, the myeloid stem cells undergo malignant transformation, becoming myeloblasts, which are types of immature white blood cells that can proliferate and not differentiate into mature blood cells.9 These myeloblasts accumulate and result in ineffective erythropoiesis and bone marrow failure. AML is categorised, according to the WHO classification system, based on morphology, immunophenotype, karyotype, molecular features, and clinical features. The WHO system has superseded the French–American–British (FAB) classification scheme.10 Several congenital disorders like Down syndrome and Bloom syndrome also predispose to AML. Besides these, environmental exposures like radiation, tobacco smoke, benzene, and exposure to chemotherapeutic agents are also risk factors for AML. However, most AML cases develop de novo in an otherwise previously healthy individual.11
Clinical features and overlap with COVID-19
A patient with AML usually presents with signs of ineffective erythropoiesis and bone marrow failure, like anaemia and thrombocytopenia, including fatigue, anorexia, and excessive bleeding. Besides this, patients can also have recurrent infections, headaches, and bone pains, as well as bruise easily. Depending on the degree of anaemia, they can experience generalised weakness, fatigue, shortness of breath, and chest tightness. Disseminated intravascular coagulation is also common in a subset of patients with AML. Lymphadenopathy and organomegaly are not very common in these patients. If not intervened, death occurs within a few months of diagnosis due to infection and bleeding.6
COVID-19 is an ongoing pandemic caused by SARS-Cov-2, a zoonotic β-coronavirus, like Middle East respiratory syndrome and SARS.12 Most of the cases are mild, with symptoms such as fever, fatigue, and a dry cough; some are severe, complicated by ARDS. Unlike the otherwise healthy population, patients with advanced age and an immunocompromised status are at a higher risk of fatal infections. This raises concern for patients with haematological malignancies like myeloid neoplasms, including AML, myelodysplastic syndromes, myeloproliferative neoplasms, and overlap disorders with features of both myelodysplastic syndromes and myeloproliferative neoplasms.8 Recipients of haematopoietic cell transplantation and cellular therapy are also at risk.13,14
Patients with AML infected with COVID-19 impose a tremendous diagnostic and treatment challenge. There are various points of concern. The striking similarity in the symptoms like fever, cough, and malaise creates confusion in diagnosis.15
The diagnosis of AML is confirmed by the presence of 20% or more blasts in bone marrow or peripheral blood. Besides this, the presence of Auer rods, and immune-phenotyping and documenting myeloperoxidase activity in cells further help establish the diagnosis.7
Treatment
Treatment consists of two phases. The first is induction therapy, which aims to kill leukaemia cells in the blood and bone marrow. The second is post-remission therapy, which kills remaining leukaemia cells, preventing relapse. Four types of treatment include chemotherapy, radiotherapy, stem cell transplant, and targeted therapy.
Complications
AML treatment is usually complicated by infections, which are important causes of morbidity and mortality. This is because of immunosuppressive state, polypharmacy, and complex epidemiology of the resistant organisms16 and can lead to treatment withdrawal. Infections are mainly haematogenous, which can be Gram-negative or Gram-positive, pulmonary, and fungal. The infection can be either documented clinically, microbiologically, or sometimes may just present as fever of unknown origin.17 Kebudi et al.18 conducted a study in children and concluded that haematologic malignancies and mixed infections (other bacterial and fungal infections) are prognostic factors for critical disease, as in this patient.
Challenges during the pandemic
Due to rapidly rising cases of COVID-19, hospitals are overburdened, leading to a shortage of isolation beds and blood products that are needed by patients with leukaemia. Prognosis in young, low-risk patients may be affected due to delays in the initiation of chemotherapy. Patients with leukaemia undergoing haematopoietic stem cell transplantation can face life-threatening complications if infected with COVID-19 and if the disease is severe. Also, giving chemotherapy in the background of COVID-19 infection looks like it could be relatively high-risk as well.
Case management
The case reported here was diagnosed with AML in January 2020 and underwent chemotherapy. He was started on induction therapy with daunorubicin and cytarabine, which have been cornerstones of remission therapy in patients with AML.19 However, due to the increasing demand for inpatient beds during the pandemic, lower intensity regimens have been developed that can be given in outpatient settings.20 Consolidation or post-remission therapy with high-dose cytarabine should continue to be offered to patients in complete remission, preferably with fewer cycles (three instead of four) and lowering the dose of cytarabine to 1.5 g/m2 instead of 3.0 g/m2.
Clinical management
The patient presented with a respiratory infection and the diagnosis of COVID-19 was confirmed by a RT-PCR test.21 The patient was initially hypoxic, with a peripheral SpO2 of 85–88% and PaO2 of 58 mmHg, which improved to 93% on 15 L/min oxygen after using a NRBM. An initial chest X-ray revealed diffuse bilateral infiltrates without cardiomegaly, consistent with ARDS, likely secondary to viral pneumonia. Due to the high infectivity rate and associated leukaemia, the patient was kept in isolation in the intensive care unit. Patients with severe COVID-19 symptoms are prone to infections, so a necessary workup was done. The authors did a complete blood count every third to fourthday. Although the procalcitonin was normal and blood cultures were negative, raised C-reactive protein and leucocytes count initially suggested underlying sepsis.
Patients with leukaemia are reported to have a higher risk of invasive fungal infections due to immunocompromised state, chemotherapeutic drugs, unfavourable cytogenetics, relapsed or refractory disease, and prolonged myelosuppression.22 Among all haematological studies, AML has the highest risk of fungal infections.23 In this patient, an underlying pulmonary infection is further predisposed to this risk. Therefore, a prophylactic antifungal, voriconazole, was added to the treatment as the best available option for the patient and cost-effectiveness.24 As per the RECOVERY trial, dexamethasone at 6 mg once daily, given intravenously, decreases mortality in patients with COVID-19 on oxygen support.25
Defer intubation
Although this patient met the criteria of severe ARDS with a PaO2/FiO2 ratio <100,26 intubation was avoided as airway reflexes were preserved, and his PaO2 was maintained at >90% on NIV and NRBM. Intubation and mechanical ventilation can cause a high risk of viral transmission to healthcare workers; besides this, there are high chances of procedure-related complications and cross-infection.27 There is also high mortality in intubated patients. Monitoring of serial arterial blood gases for pH, PaO2, and partial pressure of carbon dioxide levels was also done.
Prone positioning
Regular prone positioning was ensured in the patient for 12–16 hours daily. Prone positioning ventilation is generally used for patients with ARDS to prevent ventilator-induced lung injury. The basic mechanism involves decreasing the heart’s compressive force by acting on the lung’s dependent region, thus increasing aeration in the dorsal lung regions. There is an overall improvement in lung ventilation from dorsal to ventral areas, which are more homogeneous in the prone position than supine, thus improving oxygenation. However, perfusion remains almost constant in both postures. This physiological basis behind a prone position in ventilated patients should also apply to spontaneously breathing patients. Unfortunately, not much literature is available for prone positioning of awake patients.28
In the authors’ case, the patient was awake and spontaneously breathing but was in severe hypoxaemic respiratory failure, and prone positioning improved oxygenation, which was assessed by blood gas analysis and oxygen saturation. Early prone positioning in patients with raised inflammatory markers (increased serum C-reactive protein) is beneficial since the lungs in the initial phase of ARDS are potentially recruitable unlike in the later phase.29 Also in recent studies, it has been shown that prone ventilation combined with a non-invasive ventilator support has better effects on a ventilation–perfusion mismatch and better lung drainage in infection-induced ARDS.30
Intensive monitoring
The patient was strictly monitored for emergency signs like obstructed or absent breathing, central cyanosis, shock, coma, or convulsions. He was kept on NIV and NRBM alternatively and titrated flow rates for FiO2 levels to reach the target SpO2 of ≥93%. Once the patient maintained the target saturation with no further deterioration, the reservoir bag (at 10–15 L/min) was combined with a face mask at 4–6L/min.
Lung imaging: the new gold standard
Lung imaging is an important tool in COVID-19, for diagnosis as well as monitoring and discharge assessment. In the reported patient, a chest HRCT revealed bilateral extensive ground-glass opacities and a high CT severity score, consistent with typical findings of COVID-19 infection. COVID-19 pneumonia generally manifests on lung CT scans as bilateral, sub-pleural, ground-glass opacities with air bronchograms, ill-defined margins, and a slight predominance in the right lower lobe.31 The CT severity score is an important tool to detect the severity of COVID-19 and it provides details about the extent of lung opacities and disease burden.32
Immunosuppression and COVID-19
Minotti Chiara et al.33 published a systematic review that studied the current data on SARS-Cov-2 cases in children and adults with immunosuppression. They concluded that patients with COVID-19 who are immunosuppressed are few as compared with the overall figures, and have a favourable outcome as compared with other comorbidities. But this study drew conclusions based only on the systematic review, without a meta-analysis.33 Therefore, Gao et al.34 conducted a systematic review and meta-analysis, concluding that immunosuppression and immunodeficiency are associated with increased risk of severe disease and mortality in patients with COVID-19.
Thrombo-prophylaxis
COVID-19 is a state of profound inflammation due to the high surge of cytokines and macrophages, leading to an increased risk of thromboembolism.35 As per the recommendations, it is important to add appropriate thrombo-prophylaxis for a severe COVID-19 infection for a better outcome.36 Mechanical prophylaxis along with low-molecular-weight heparin was added while keeping a regular check on platelet count, considering the background haematological malignancy.37
Blood sugars
The authors reported inadvertently high blood glucose levels in the patient, who was recently diagnosed with Type 2 diabetes mellitus. As per the literature, there is increased risk and severity of infection in patients with diabetes who are infected with COVID-19, probably due to overexpression of angiotensin-converting enzyme receptors, increased IL-6 levels, and impaired T cell function.38 The exact mechanism is not known.
Viral clearance
Factors like advanced age, being male, and diabetes may adversely affect viral clearance.39 This may explain the repeatedly positive RT-PCR test in the authors’ patient, which came negative in the fifth sample. In addition to this, previous studies also suggest that in patients with AML, glycaemic variability is associated with poor outcomes and lower remission rates.40 Hence, these patients need more attention for adequate control and management of hyperglycaemia.
Mental health
COVID-19 has a major impact on patients’ mental health, who face a lot of emotional adjustment, partly due to the disease itself and partly because of the isolation accompanying it.41 Along with clinical management, the psychological health of patients’ needs attention. Patients need help to maintain a positive attitude to overcome stress, anxiety, and panic associated with the disease. The authors regularly counselled and motivated their patient regarding the prognosis and outcome of the illness. The family was allowed to contact the patient on phone through video calling while maintaining all standard precautions. Music therapy was also provided.
Home isolation
After approximately 1 month, the patient was discharged as per the criteria and was advised to strict home isolation and pulse oximeter monitoring for domiciliary oxygen therapy. A chest CT scan, nasopharyngeal swab, and clinical examination were repeated 15 days after the patient was discharged. He was advised to continue oral voriconazole 200 mg twice daily for 2 weeks.
LIMITATION
A limitation of the study was the non-availability of the galactomannan test in the authors’ hospital. However, follow-up radiological imaging did not suggest any findings of invasive fungal infection.
CONCLUSION
To conclude, the authors reported a case of COVID-19 occurring in a patient with AML with a good outcome. Patients with haematological malignancies are more susceptible to SARS-CoV-2 infection and further development of severe infection, including pneumonia with poor blood oxygenation. COVID-19 infection can cause significant harm to a patient with underlying leukaemia, increasing mortality risk. The management of such patients is more challenging than expected, especially in patients with superimposed bacterial infections. The importance of supportive management (oxygen with a bilevel positive airway pressure, prone positioning, and physiotherapy) to prevent complications is emphasised. It also has a significant impact on the physical and psychological health of the patient, necessitating special care and attention. Due to a lack of sufficient literature, more research is needed on the treatment and management strategies for patients with leukaemia during the COVID-19 pandemic.






