Abstract
Neonatal sepsis is a major cause of morbidity and mortality in newborns. It presents a diagnostic challenge to the neonatologists due to a lack of objective evaluation. It may mimic noninfective conditions, such as inborn error of metabolism, birth asphyxia, and even respiratory distress syndrome in preterms. Nonetheless, over-diagnosis and initiating unwanted empirical antibiotics may pose the threat of drug resistance, increasing the hospital stay and cost of treatment. Traditionally, investigations such as white blood cell count, absolute neutrophil count, immature to total neutrophil ratio, C-reactive protein levels, and blood cultures have been used to diagnose sepsis. However, these have low sensitivity and specificity because they may be elevated in conditions other than sepsis. The in-depth understanding of the neonatal immune system’s response to early infection has led to the discovery of advanced diagnostic tools, including biomarkers.
This literature review briefs on the various haematological parameters and biomarkers in neonatal sepsis, exploring newer biomarkers and comparing them with their older counterparts. This will help early diagnosis, treatment, and improved prognosis in neonatal sepsis. As there is a spectrum of markers for diagnosing neonatal sepsis, it is preferable to compile these markers and correlate clinically.
A thorough search of this literature was done on the electronic databases PubMed, Elsevier’s Web of Science, and the Cochrane Library. The authors found around 90 relevant articles: 84 were from PubMed, 4 from Elsevier, and 2 from the latest Cochrane database. Of these articles, 57 were selected from between early 2000 and January 2019.
INTRODUCTION
Neonatal sepsis has been a leading cause of high morbidity and mortality in newborns and is recognised as a global health challenge.1-3 The definition of early onset sepsis (EOS) is variable from <3 days (American Academy of Pediatrics [AAP] definition) to <7 days (Centers for Disease Control and Prevention [CDC] definition based on epidemiology studies).4 The incidence of neonatal sepsis in India was 30/1,000, as per the Neonatal Perinatal Database (NNPD).5 The incidence of total sepsis was as high as 14.3% in a recent cohort study conducted by the Delhi Neonatal Infection Study (DeNIS) collaboration in India, of which culture-proven sepsis was 6.2%. Nearly two thirds of these cases were EOS (<72 hours).6 The total neonatal mortality rate was 28/1,000 live births with early neonatal mortality rate being 22/1,000 live births, of which sepsis contributed to a quarter of the deaths.7 Therefore, neonatal sepsis constitutes a significant health burden. To combat this, it is vital to understand the fragile neonatal immune system and its response to infection in the form of biomarkers.8 Timely identification of neonatal sepsis and initiation of appropriate antibiotics form the cornerstone in preventing these neonatal deaths.
Even though there are recent sophisticated biomarkers to diagnose sepsis, it is still challenging to curtail sepsis mortality by timely intervention.9-11 Moreover, establishing early diagnostic markers will extensively reduce antibiotic abuse and help in rationalising a unit policy for the judicious use of antibiotics, thus preventing the emergence of multidrug-resistant micro-organisms. The current gold standard microbiological blood culture screen may produce false-negatives because of low yield when a lesser amount of blood is collected, in addition to high turnover time.12,13 Additional haematological tests used traditionally, such as white blood cell count (WBC), absolute neutrophil count (ANC), immature to total neutrophil (I/T) ratio, and C-reactive protein (CRP) measurement, also have a poor sensitivity and specificity and may need serial monitoring.14 For better prediction of sepsis, recent evidence suggests the use of age-specific nomograms rather than fixed normal ranges of WBC, ANC, and I/T ratio.15 Thus, diagnostic tests that are rapid and accurate in guiding the management of septic newborns are needed.16 Empiric use of unnecessary antibiotics will be withheld by the use of tests that have a high negative predictive value, therefore preventing the adverse effects of antibiotics on unaffected neonates.17,18 The immune system of a neonate remains incompletely understood; however, with the robust development of molecular characterisation of this immature immune system, it has now been possible to identify an array of biomarkers that are produced by these ill infants in response to the offending organism aiding the prompt diagnosis of sepsis.19-21 The objective of this review is to provide a summary of biomarker developments for early diagnosis and treatment of neonatal sepsis, simultaneously comparing them with older markers for the condition.
METHODS
The authors completed a computer-based search of the literature using the words “neonatal”, “sepsis”, “biomarkers”, “hematological”, and “omic”, along with combinations of these words in PubMed, Elsevier’s Web of Science, the Cochrane Library (including January 2019), and Google. The results revealed around 90 studies in the English language from early 2000 to January 2019; most of which were single-centred, small studies conducted in tertiary care neonatal units in middle and low-income countries. Of the 57 articles included in the review, most of them were cohort, cross-sectional studies, and previous reviews and articles retrieved from their references. This was followed by detailed, extensive analysis of these studies elaborating each of the biomarkers including both the traditional and newer ones (Table 1), comparing them (along with their sensitivity and specificity), and describing their practical utility in a resource-limited set up.
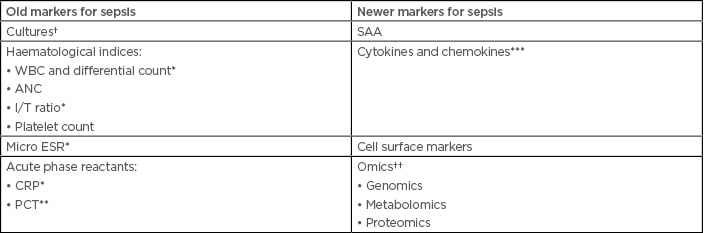
Table 1: Old and new biomarkers for the diagnosis of sepsis.
*Forms the conventional septic screen. **Excellent early marker, especially if combined with PSP, thus forming the best marker for EOS. ***IL-6 early biomarker in EOS. †Gold standard. ††Promising markers to be explored. ANC: absolute neutrophil count; CRP: C-reactive protein; EOS: early onset sepsis; I/T ratio: immature/total neutrophil ratio; Micro ESR: micro erythrocyte sedimentation rate; PCT: procalcitonin; PSP: pancreatic stone protein; SAA: serum amyloid A; TLC: total leukocyte count; WBC: white blood cell count.
*Forms the conventional septic screen.
**Excellent early marker, especially if combined with PSP, thus forming the best marker for EOS.
***IL-6 early biomarker in EOS.
†Gold standard.
††Promising markers to be explored.
ANC: absolute neutrophil count; CRP: C-reactive protein; EOS: early onset sepsis; I/T ratio: immature/total neutrophil ratio; Micro ESR: micro erythrocyte sedimentation rate; PCT: procalcitonin; PSP: pancreatic stone protein; SAA: serum amyloid A; TLC: total leukocyte count; WBC: white blood cell count.
THE REQUIREMENTS OF AN IDEAL BIOMARKER
- Levels of the biomarker should aid not only in early diagnosis but also in optimising management. Thus, biomarker levels should change early in the disease and remain altered for a period of time.8
- High sensitivity and negative predictive value of nearly 100%, with a preferable specificity and positive predictive value of 85%.9,22
- Guide in starting and/or stopping antimicrobial treatment and also monitor disease course.
- Discriminate a specific pathogen or a category of pathogens, e.g., viral, bacterial (gram-positive organisms versus gram-negative organisms), and fungal organisms.
- Be able to predict severity of the disease as well as prognosis.
- Volume of specimen needed should be small (e.g., <0.5 mL of blood), low cost, and readily available.
- Quantitative values of biomarker concentration with nomograms or well-defined cut-offs must be easily available.
OLD MARKERS
Cultures
The definitive diagnosis of sepsis is the isolation of the organism from any bodily fluid, such as blood, urine, or cerebrospinal fluid. Although the sensitivity of blood cultures is 98%, results normally take up to 72 hours. Furthermore, prior antibiotic treatment and low volume of collection can result in false-negative reports. Nevertheless, BACTEC culture system has now made early detection within 48 hours possible, even with low volumes of blood and a low colony count.23
Haematological Indices
Traditionally, the following parameters have been used as the initial markers of neonatal sepsis, either individually or in combination:
- Total leukocyte count (TLC).
- ANC.
- I/T ratio and morphological or degenerative changes in neutrophils, such as vacuolisation, Döhle bodies, intracellular bacteria, and toxic granules.
- Platelet count.
Total Leukocyte Count
TLC has been conventionally used in the sepsis screen. Based on the fact that there are few reserves of white blood cells in the neonatal bone marrow, leukopenia can represent an overwhelming infection.24,25 However, TLC has a poor predictive value in diagnosis of EOS. Moreover, in EOS, neutrophil indices are more reliable if obtained after 6–12 hours, thus delaying the diagnosis.
Absolute Neutrophil Count
Blood ANC varies in a neonate, with the lower limit being <1,800/µL at birth, <7,800/µL at 12–14 hours of age, and falling again to <1,800/µL at 72 hours.
Immature to Total Leukocyte Ratio
I/T ratio is calculated as ‘immature polymorphs/mature plus immature neutrophils’ and is the most sensitive indicator of sepsis. Values >0.27 in term and >0.22 in preterm neonates are significant. In general, the abnormal leukocyte ratios, including an I/T ratio of ≥0.2, tend to have a high sensitivity of 90% and negative predictive value of 98%, whereas abnormal leukocyte counts, such as leukopenia and neutropenia, tend to have high specificity.
Platelet Count
Thrombocytopenia has been seen quite often in sepsis, especially fungal sepsis, but has not been a promising early marker.
The haematological scoring system (HSS) suggests that the higher the score, the greater the sensitivity, and that sepsis is probable with a HSS score ≥3. This test has a high sensitivity of 96%, but a low positive predictive value of 31%. Although it is a complex scoring method, studies have complied HSS data with biomarker usage to obtain better results.26
Increased mean platelet volume (>8.6 fL) has been studied recently as a marker of EOS and a predictor for mortality, especially in preterm neonates with a sensitivity of 97.14% and a specificity of nearly 100%. However, elevated levels of this marker may also be seen in bronchopulmonary dysplasia and respiratory distress.27 In addition, recent studies have shown that increased (20%) red blood cell distribution width within 6 hours after birth has been associated with EOS and also predicts a poor outcome.28
Micro Erythrocyte Sedimentation Rate
Micro erythrocyte sedimentation rate (Micro ESR) is a technique that has been used traditionally in the septic screen; however, it lacks sensitivity because it takes a few days for ESR to rise and the value varies significantly within the first few days of life. Furthermore, the levels take a significant amount of time to return to normal again. A rough estimate of calculation can be achieved through permiting an additional 2 or 3 days to the age of the neonate being tested.29
Acute Phase Reactants
C-Reactive Protein
CRP, a component of the septic screen, is an acute phase reactant produced by the liver in response to an inflammatory/infectious process. It also helps to predict disease severity and guide the antibiotic duration.30 CRP levels increase at least 6 hours after onset of acute inflammation and decrease faster than any other acute phase reactant.31 Rather than a single value, serial monitoring at 24 and 48 hours after the onset of sepsis improves sensitivity (by 82% and 84%, respectively). However, CRP may be elevated post-surgery, in meconium aspiration cases, and in those who have recently had vaccinations, thereby reducing its specificity. Since CRP may not be increased immediately at the onset of sepsis, other biomarkers are highly warranted for timely initiation of the treatment. In preterms, for whom CRP production may not be sufficient, the high sensitivity assays of CRP (hs CRP) form a vital marker because it can detect an even lower grade of inflammation.32 A recent review concluded that serum CRP levels at initial evaluation in suspected late-onset infection is neither sufficiently accurate for early diagnosis nor selecting of neonates that need further investigation and antimicrobial therapy.33
Procalcitonin
Procalcitonin (PCT) is the prohormone of calcitonin and is produced by monocytes and hepatocytes in response to sepsis. In contrast to CRP, PCT levels rise 2 hours after infection, peak at 6–8 hours, and normalise after 2–3 days.34 Although levels are unaffected by gestational age, specific nomograms need to be referred to for the reference ranges in early days of life. PCT has higher sensitivity compared to CRP and other biomarkers, such as IL-6 and hs CRP, especially in early detection of infection.35 PCT in early-onset infection has been reported to have a sensitivity of 92%, specificity of 97%, positive predictive value of 94%, and negative predictive value of 96%.36 As evidenced by meta-analyses with pancreatic stone protein (PSP), PCT forms the best marker for EOS.37 Nonetheless, false-positive increases in PCT may be seen at times in neonatal hypoxia and intracranial bleeding.38
NEW MARKERS
Serum Amyloid A
Serum amyloid A (SAA) is an early acute phase reactant and is produced in the liver as apolipoprotein (Apo) SAA in response to infection and inflammation.39 However, the hepatic and nutritional status may affect the values, limiting SAA’s use in late-onset sepsis.40 SAA has not only proved to be a more sensitive and specific marker, with sensitivity and specificity of 96%, but has also helped in prognosticating patient mortality.41,42
Cytokines and Chemokines
Among the various cytokines released by the immature neonatal immune system, the major ones are TNF-α, IL-6, and IL-8.10 IL-6 is a proinflammatory marker synthesised by mononuclear, chorion, amnion, and trophoblastic cells, and proves to be an early marker in sepsis. This protein triggers the production of CRP and thus its levels elevate prior to CRP. The disadvantage, however, is its short half-life.43,44 TNF-α, a proinflammatory cytokine, stimulates IL-6 production but is not as sensitive as IL-6 itself.23 It has been studied that the combination of IL-6, TNFα, and CRP has a sensitivity and negative predictive value of approximately 90% for diagnosing EOS.45 The proinflammatory cytokine IL-8 mediates leukocyte migration and activation and its level rises and falls within 4 hours of infection; it also has a sensitivity of 90%, and has a varied specificity between 75–100%.46
Cell Surface Markers
In response to sepsis, various inflammatory cells express cell surface markers, such as CD11b, CD116, CD64, and CD45RO, which can be detected by flow cytometric analysis. This is useful in diagnosing intra-abdominal infections as well as EOS. Amongst these, CD64 plays an important role because it binds to the Fc region of the immunoglobulins that increase in infection. The sensitivity of CD64 in diagnosing EOS is 80% and negative predictive value is 89%;47 however, combined with CRP and IL, its sensitivity may reach 100%. Another new, promising marker is soluble CD163 in EOS with sensitivity up to 100%. In addition, it helps to differentiate between infectious and non-infectious conditions.48,49
‘Omics’ as Future Markers
The various newer approaches in ‘omics’ include the following:
- Genomics
- Metabolomics
- Proteomics
Genomics
Micro-organisms’ genomes, including their response to infection, can be studied, forming the basis of microbial genomics. PCR plays a key role in this process. Even though PCR takes longer to detect organisms, it has been found to be useful in diagnosing viral as well as fungal infections.50 However, quantitative PCR (qPCR) can rapidly detect any infection. By using the probe specific qPCR, many bacterial species can also be identified. The limitation to this, however, is the non-availability of the entire genome of the microbiome and resistant cell walls of some bacteria, making the DNA unavailable for sequencing.51
Metabolomics
The metabolites produced by the micro-organisms in response to sepsis can be studied by spectrometric analysis (such as magnetic resonance spectrometry, nuclear magnetic resonance, and gas-chromatography mass spectrometry), reflecting the interaction between environment and the gene. Apo C2 and SAA were found to be especially elevated in necrotising enterocolitis and hence Apo SAA score was made in a study by Ng et al.12 Further studies are warranted for the wide acceptance of this score. However, compiling this core can be time consuming, thus it may not be appropriate in EOS. Nevertheless, it has a wide scope in the future to explore the interactions between host and defence and would enable the identification of more biomarkers for septicaemia.52,53
Proteomics
Similar to genomics, exploring the proteins produced by the immune system’s defences in response to organisms can be evaluated using proteomics. One example is S-100 (calgranulin), which along with heat shock proteins and altered matrix proteins, forms a part of the group of proteins called damage associated molecular proteins. These are produced by the fetus in response to inflammation and have a protective role. Buhimschi et al.54 generated a mass restricted score on the amniotic fluid to predict EOS and found elevated levels of matrix metaloprotease-8 (MMP-8) in the amniotic fluid of mothers with prolonged rupture of memebrane. Similarly, another study had reported increased levels of IL-8 mRNA expression in neonates exposed to perinatal infection.55
To summarise, these modern molecular markers are not only definitive, but also rapid in diagnosing neonatal infections (Table 2).56
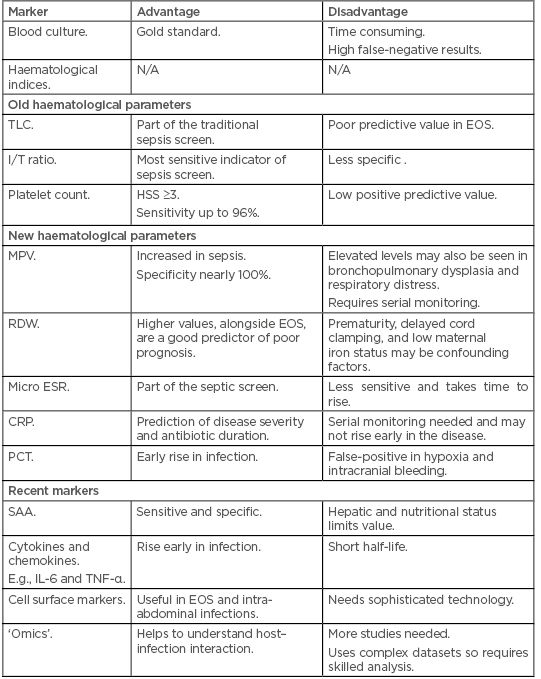
Table 2: The advantages and disadvantages of old and new haematological markers and other new biomarkers for the diagnosis of sepsis.
CRP: C-reactive protein; EOS: early onset sepsis; HSS: haematological scoring system; I/T ratio: immature to total neutrophil ratio; Micro ESR: micro erythrocyte sedimentation rate; MPV: mean platelet volume; N/A: not applicable; PCT: procalcitonin; RDW: red blood cell distribution width; SAA: serum amyloid A; TLC: total leukocyte count.
MORE MARKERS IN THE PIPELINE
Flow cytometric analysis of various body fluids may be helpful in early diagnosis. Among them are IL-8, inducible protein (IP10), and MCP-1. The sensitivity and specificity of IP10 plasma values of ≥1,250 pg/mL are 93% and 89%, respectively, in neonatal sepsis, especially in preterms.57
Markers for Early Onset Sepsis
CRP, PCT, and PSP in combination with the traditional markers are markers for EOS, as mentioned in this review. Other markers for EOS are lactoferrin, alpha-1-acid glycoprotein, haptoglobin, and neopterin. In addition, visfatin and resistin have been used similarly for diagnosing EOS, along with chemokines and cytokines.
Markers for Late Onset Sepsis
SAA, ischaemia-modified albumin, and hepcidin are the newer markers advocated in late onset sepsis. They can be combined with the old markers of sepsis to yield a better result in diagnosing late onset sepsis.58
CONCLUSION
Though ample new markers have been explored, much of the population in developing countries may not be able to afford them, because they require sophisticated technology. In such situations, PCT would be an apt marker, especially in EOS, thus aiding in timely intervention and reducing the mortality. This is extremely crucial in low and middle-income countries where EOS is fulminant and is caused by gram-negative micro-organisms leading to early mortality. Despite being costly, the governing bodies of such nations should reconsider the need of including these modern biomarkers in the septic screen of these fragile neonates, as they are more specific and help in prompt diagnosis. This, in turn, would reduce the overall burden on the healthcare system and would therefore prove to be more cost effective in the long run due to curtailing the cost of prolonged hospital stays, mortality, and morbidity burden. In conclusion, despite of an array of biomarkers being available, it is crucial to select the appropriate combination of these markers that allows only minimal blood loss in the neonate for a timely, precise diagnosis of sepsis.







