Abstract
Introduction: Polycythaemia vera (PV) treatment focuses on preventing thrombotic events and delaying transformation to myelofibrosis or leukaemia. According to risk stratification, low-risk patients require therapeutic phlebotomy combined with acetylsalicylic acid, whilst the treatment of high-risk patients with PV relies on cytoreductive therapies, employing hydroxyurea (HU), ruxolitinib, or interferons. However, in low- and middle-income countries, the availability and cost of these drugs poses a challenge in treating high-risk patients, so optimising existing resources is required.
Method: A prospective longitudinal study aimed to investigate the combination of atorvastatin (ATV), aspirin, and low-dose HU as a therapeutic strategy to treat PV in high-risk patients. The study evaluated the effect of statins on erythroid colony proliferation in vitro, as well as the applicability of ATV (20 mg/day), acetylsalicylic acid (100 mg/day), and hydroxyurea (500 mg/day) in high-risk patients with PV from La Paz, Bolivia, residing at 3,600 metres above sea level.
Results: Simvastatin (3.5 μm) inhibited UKE-1 cell (JAK2V617F mutated) proliferation at 33%, and burst-forming unit-erythroid colonies from patients with PV at 61%. Patients receiving ATV, aspirin, and low-dose HU displayed a good response and adequate tolerance to treatment (13-years follow-up). No patients experienced myelofibrosis or transformation to leukaemia, and no severe adverse events were observed.
Conclusions: This accessible, effective, and low-cost therapeutic strategy could improve adherence to treatment and the overall survival of high-risk patients with PV in resource-limited countries.
Key Points
1. Cost and availability barriers to treatments for polycythaemia vera (PV) that prevent thrombotic events and delay transformation to myelofibrosis or leukaemia affect patients in low- and middle-income countries.2. Combination treatment with atorvastatin, aspirin, and low-dose hydroxyurea demonstrated good response (cytoreduction, inhibition of erythroid proliferation, and prophylaxis of thrombosis) in a prospective, longitudinal study of 14 high-risk patients with JAK2[sup]V617F[/sup] mutations and PV over 13 years follow-up.
3. Lower cost, higher availability, effective treatments could improve adherence to treatment and the overall survival of high-risk patients with PV in resource-limited countries.
INTRODUCTION
Polycythaemia vera (PV) is a clonal myeloproliferative neoplasm that is characterised by the JAK2V617F mutation in more than 95% of patients. The overall median survival is 13.5 years,1 but risk stratification in PV can identify high-risk patients with a median survival of 10.9 years, intermediate-risk patients with a median survival of 18.9 years, and low-risk patients with a median survival of 27.8 years.2 The primary goals of current PV therapies are preventing thrombotic events and delaying transformation to myelofibrosis (MF) or acute myeloid leukaemia.3,4
Treatment for PV aimed at maintaining haematocrit or haemoglobin (Hb) levels within the normal range has been associated with a reduction in cardiovascular deaths and thrombotic events.5-7 Currently, low-risk patients with PV (<60 years and without previous thrombotic events) are treated with acetylsalicylic acid (ASA) and phlebotomy, whereas high-risk patients with PV require additional cytoreductive therapy, generally with hydroxyurea (HU), interferon-α, or ruxolitinib.8-11 In the low- and middle-income countries of Latin America, high-risk patients with PV have difficulty acquiring treatment due to the high cost and lack of availability of drugs; therefore, optimising existing resources to provide more accessible, adequate, and effective treatment protocols should be considered.
Atorvastatin (ATV) is a common drug used in clinical practice. It has pleiotropic effects in erythroid proliferation and differentiation, as well as in the prevention of thrombotic events, and sensitises cells to HU’s mechanism of action, thus allowing for lower doses to be used.12-16 As part of the treatment protocol for PV, ASA works as an antiplatelet agent to prevent thrombosis, and also acts as an inhibitor of the nuclear factor NF-κ-B p105 (NFKB1) protein,17-20 an activator of hypoxia inducible factor (HIF)-1α.21 Down regulation of NFKB1 by ASA may decrease erythropoiesis by decreasing HIF-1α activity.22,23 HU, in turn, is a well-known cytoreductive drug in the treatment of PV.24-26
The combination of the aforementioned drugs may be an alternative for treating high-risk PV in settings of limited economic resources, improving both adherence to treatment and overall patient survival. This study aimed to investigate the combination of ATV, ASA, and HU as an accessible and low-cost therapeutic strategy for high-risk PV.
METHODS
In Vitro Assays
Assays of erythroid colony proliferation in vitro, with or without simvastatin (3.5 μm) were performed in cell lines (UKE-1 and K562) and bone marrow mononuclear cells obtained from patients with PV, as well as from healthy donors (normal controls) as previously described.27,28 The assays were focused on showing the effect of simvastatin on neoplastic cells, since HU cytotoxic action and ASA activity are well known.
Patients
After human research committee approval, a prospective longitudinal study was conducted from January 2008 to April 2020. Of a total of 91 patients diagnosed with PV, 14 high-risk patients with JAK2V617F with PV were evaluated. All treated patients were high-altitude dwellers (>3,600 meters above sea level) from La Paz, Bolivia, where normal Hb levels in healthy subjects range from 15–18 g/dL in males and 14–17 g/dL in females.29 The diagnosis of PV was made according to standard diagnostic criteria,30 and risk stratification was performed by the Griesshammer algorithm, in which ‘high-risk’ refers to patients 60 years of age or older and/or with a previous thrombosis.31,32 Laboratory studies (blood count, blood glucose, uric acid, creatinine, bilirubin, transaminases, lactate dehydrogenase, and erythropoietin) were performed, and thrombotic events were confirmed by echo-Doppler and computed tomography. Patients included in this study did not have previous treatment with other medications.
Evaluation of the JAK2V617F Mutation
Evaluation of the JAK2V617F mutation was performed through a PCR assay using the common reverse primer JAK2 R (5′-CTGAATAGTCCTACAGTTTTCAGTTTCA-3′) at 50 μM and two forward primers at 25 μM, namely JAK2 F mutation (5′-AGCATTGGTTTTAAATTATGGAGTATATT-3′), specific for the mutant allele, and JAK2 F WT (5′-ATCTA-TAGTCATGCTGAAAGTAGGAGAAAG-3′), which amplifies the wild-type allele. PCR was performed at an annealing temperature of 59 °C for 35 cycles. Amplification resulted in a 203-base pair product for the mutant allele and a 364-base pair product for the wild-type allele.27,28
Treatment
Phlebotomies of 450 mL were performed in patients until Hb levels were <17 g/dL in females and <18 g/dL in males. Then, after informed consent, high-risk patients with PV received HU (500 mg/day), ATV (20 mg/day), and ASA (100 mg/day) orally. A monthly outpatient follow-up was carried out. Possible adverse events related to each drug were monitored. The dose of HU was adjusted as needed to maintain neutrophils >2,000/μL and platelets >100,000/μL. The dose of ATV was reduced for muscle pain or an increase in creatine phosphokinase. The dose of ASA was modified if bleeding occurred, particularly epistaxis, gingivorrhagia, or petechiae.
Statistical Analysis
Descriptive analysis, using means and standard deviation, was performed through GraphPad Prism Version 6.0 (GraphPad Software Inc., California, USA), and included a Student t-test to evaluate the significance of any differences in the blood count. Figures were created using Microsoft Office Excel Version 16.23.190309 (Microsoft Corp., Washington, USA). A p-value of <0.05 was considered to be significant.
RESULTS
Inhibition of Cell Proliferation
In vitro studies performed in cell lines UKE-1 (JAK2V617F mutated) and K562 (BCR-ABL positive) displayed that simvastatin (doses of 3.5–14.0 µM, added over 5 days) induced a 33–100% dose-related inhibition in UKE-1 cell proliferation, and a 5% inhibition of K562 cell proliferation at all doses (Figure 1A).
Inhibition of Burst-Forming Unit-Erythroid Colonies
Based on the results reported above, similar assays using bone marrow mononuclear cells from PV patients and from healthy subjects as normal controls were performed. The addition of simvastatin (3 µM) induced a 61% inhibition in burst-forming unit-erythroid colonies from patients with PV and a 51% inhibition in normal controls (Figure 1B).
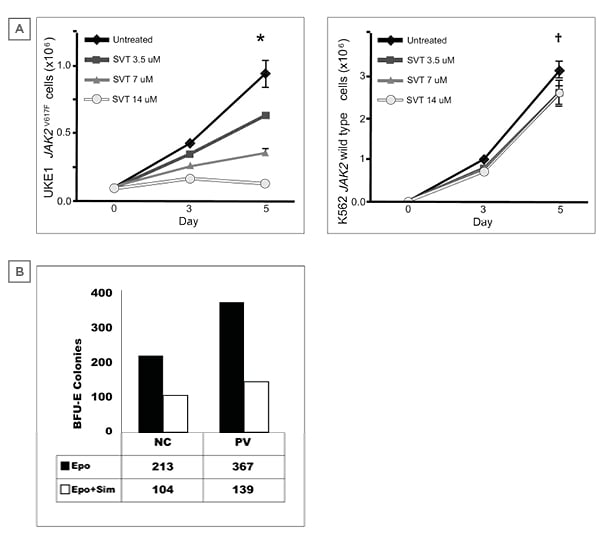
Figure 1: Inhibition induced by simvastatin.
A) Inhibition of cell proliferation in UKE1 and K562 cell lines. UKE-1: homozygous cell line with JAK2 gene mutation obtained from a patient with essential thrombocythemia. K562: Immortalised cell line from chronic myeloid leukaemia (BCR/ABL) in blast phase.
B) Inhibition of burst-forming unit-erythroid colonies with simvastatin. Methylcellulose-based semi-solid culture of bone marrow haematopoietic progenitor cells to different simvastatin concentrations.
*p<0.001
†p<0.05
‡2 UI/mL
§2 μm
**n=3
Epo: erythropoietin; NC: normal controls; PV: polycythaemia vera; Sim: simvastatin; STV: simvastatin statin.
Atorvastatin, Acetylsalicyclic Acid, and Hydroxyurea in High-Risk Patients with Polycythaemia Vera
The 14 patients at high-risk of PV included 7 males and 7 females, with an average age of 65 years. Five of them had a history of thrombosis (1 deep vein thrombosis, 3 portal vein thrombosis, and 1 stroke). Comorbidities such as diabetes (n=1), dyslipidaemia (n=1), systemic arterial hypertension (n=3), and a history of smoking (n=1) were identified. Almost all (13 out of 14 patients) had a history of previous phlebotomies. Baseline characteristics of the high-risk patients treated with ATV, ASA, and low-dose HU are shown in Table 1.
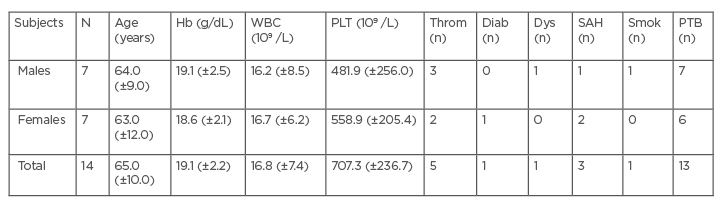
Table 1: Baseline characteristics of treated patients.
Results are displayed in mean and standard deviation (±).
Diab: diabetes; Dys: dyslipidaemia; Hb: haemoglobin; PLT: platelets; PTB: phlebotomy; WBC: white blood cells; SAH: systemic arterial hypertension; Smok: smoking; Throm: thrombosis.
Six follow-up evaluations were conducted from 6 months to 13 years (Table 2). Characteristics of response, progression, and tolerance to treatment were considered.
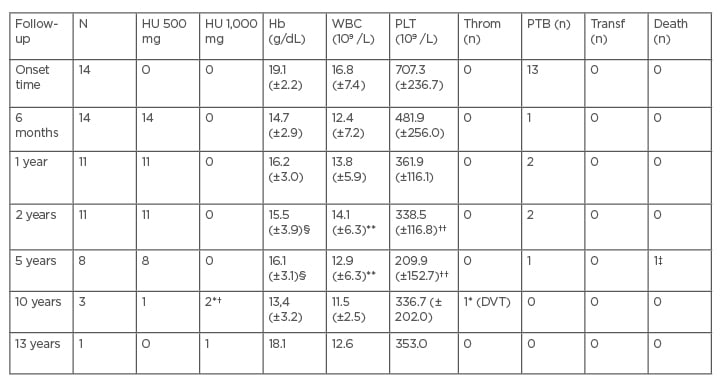
Table 2: Patients follow-up.
Results are displayed in mean and standard deviation (±). HU dosage change is depicted, there was no dose change in ATV (20 mg) and ASA (100 mg).
*A 67-year-old patient who presented DVT and splenomegaly increased HU to 1,000 mg/day, followed by rivaroxaban 20 mg daily. The patient had a good evolution.
†A 72-year-old patient who presented leukocytosis (16.9×109 /L) increased HU to 1000 mg with good evolution.
‡A 65-year-old patient with history of smoking died as a result of lung cancer.
§Hb: p<0.0077 at 2 years follow-up; p<0.015 at 5 years follow-up.
**WBC: p<0.34 (NS) at 2 years follow-up; p<0.22 (NS) at 5 years follow-up.
††PLT: p<0.0001 at 2 years follow-up; p<0.0001 at 5 years follow-up.
ASA: acetylsalicylic acid; ATV: atorvastatin; DVT: deep vein thrombosis; Hb: haemoglobin; HU: hydroxyurea; NS: not significant; PLT: platelets; PTB: phlebotomy; WBC: white blood cells; Throm: thrombosis; Transf: transfusion.
After 10 years of treatment, two patients needed to increase the dose of HU to 1,000 mg. A 67-year-old patient (10-years follow-up), who presented with deep vein thrombosis, had their dose of HU increased to 1,000 mg/day followed by rivaroxaban 20 mg daily. A 72-year-old patient (13-years follow-up), who presented with leukocytosis (16,900 /μL) had their dose of HU increased to 1,000 mg/day. Both patients had a good response, with performance statuses of 1 and 0, respectively, at the time of writing. However, a 65-year-old patient (5 years follow-up), with a history of smoking, died as a consequence of lung cancer.
The Hb, leukocyte, and platelet levels decreased progressively in patients, with statistical significance in Hb and platelet counts evaluated at 2 and 5 years of treatment (Table 2). Phlebotomies were required during up to 5 years of follow-up. None of the patients experienced progression to MF or transformation to leukaemia. No severe adverse events requiring discontinuation of treatment were observed. Leukocytes and platelets remained over 10,000/μL and 200,000/μL, respectively.
DISCUSSION
Statins are effective for inhibiting the growth and differentiation of JAK2V617F-dependent cells by altering the lipid rafts where JAK2 resides.14,33 The use of statins inhibiting cholesterol synthesis combined with JAK inhibitors may provide a more effective therapeutic strategy for patients with high-risk PV than the use of JAK inhibitors alone or the use of HU alone.34
Exposure to statins in patients with PV reduces the odds of requiring high intensity phlebotomies by 84%, possibly because statins inhibit JAK2 pathway-dependent cell proliferation.12 Statins reduce the location of JAK2 in lipid rafts, negatively regulate JAK2/signal transducer and activator of transcription 5 activation, and inhibit cell growth in cell lines containing JAK2V617F.13,14 A potential role for statins as an adjuvant treatment of patients with PV has been supported by findings that statins inhibit erythroid colony formation, contribute to JAK inhibitors (JAK1, JAK2) such as ruxolitinib, and reduce proliferation of JAK2V617F positive cells.35 In addition, the antithrombotic action of statins is relevant in PV35,36 where risk for thrombotic events is high.23,36-38 In this regard, the thrombotic problem is likely to be greater at high-altitude regions, where HIF is increased by hypoxia and the expression of several HIF-regulated thrombo-inflammatory genes increases the thrombotic risk.23,39,40 Patients with PV at high altitudes are at a particularly high risk of thrombosis due to population Hb and haematocrit levels being above the normal ranges found at sea level.41,42
ASA is recognised as an antiplatelet drug and as a NFKB1 inhibitor; in its role as an inhibitor of NFKB1, it potentially decreases erythropoiesis by inhibiting HIF-1α.43 The use of ASA in PV is safe and widely known.17,44
Regarding HU, the cytotoxic drug has been the conventional treatment for PV in high-risk patients, despite studies that employ HU alone without combining other drugs displaying few encouraging results.1,45 HU is usually well tolerated. It influences several critical factors contributing to the reduction of hyperviscosity and to blood rheology, including haematocrit, haemoglobin, and erythrocyte morphology.45-48
In this study, high-risk patients with PV who received the combination of HU (low-dose), ATV, and ASA displayed a good response and adequate tolerance to treatment. The most long-standing patient in the study has received this treatment combination for 13 years, and others have received it for at least 10 years. One case for this drug combination presented with a thrombotic event, and this was after 10 years of treatment. Only two patients had to increase their dose of HU to 1,000 mg after 10 years of treatment, and considering such a length of time, no results statistical variations were noticed. After 5 years follow-up, a favourable response to treatment was reported to be 87.5%. This is much higher than in other studies where 1,000–2,000 mg of HU alone was administered, which reported a response of 79% at 2 years follow-up49 and 63% at 4 years follow-up.50 Similarly, a good response was observed in the patients within 10 years follow-up (n=4).
The low rate of thrombosis in the authors’ study is especially striking since some reports point out that a high altitude may magnify the risk of thrombosis in patients with PV.40 None of the patients experienced transformation to leukaemia or MF. Several reports have indicated the probable leukaemogenicity of HU in PV. The reported range is from non-existent to 11.5% after 10.0 years of exposure.51-53 A leukaemic transformation rate of 0.4% after 12.4 years of exposure was recently reported,54 which supports the results obtained in this study.
It is also relevant to evaluate features such as the length of experience, cost efficiency, and safety of these drugs to assess their potential as reliable, accessible resources in settings where there is a need to reconcile economic sustainability with the right to a better quality of health and life.54 In Latin American, including Bolivia, the treatment for PV that employs ATV, ASA, and HU requires a 30 USD monthly investment, whereas treatment with interferons or ruxolitinib may imply an investment of around 600 USD and 2,500 USD monthly, respectively. It is, therefore, noteworthy that the treatment combination applied in the authors’ study was economically feasible, which enabled a good adherence to the regimen.
Future studies are needed to measure JAK2V617F before and during therapy in order to determine the effectiveness of HU in reducing the burden of this allele. This is necessary as part of the studied combination of drugs at high altitude, since previous studies in other populations have provided conflicting results.55 Likewise, ATV could play a beneficial role in other clinical situations of PV, especially in patients with resistance to HU,56 and patients with contraindication to phlebotomy.57
CONCLUSION
In conclusion, the combination of HU, ASA, and ATV has effects of cytoreduction, inhibition of erythroid proliferation, and prophylaxis of thrombosis in patients with PV. This combination potentially provides patients from low- and middle-income countries with an accessible, low cost, and effective treatment for high-risk PV.






