Interview: Richard Cowan
Cancer Research UK, The Christie NHS Foundation Trust, Manchester, UK
Disclosure: The interviewee has acted as a consultant for Kyowa Kirin International.
Acknowledgements: Writing assistance was provided by Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the named author.
Support: The writing and editorial support were funded by Kyowa Kirin International.
Citation: EMJ Hematol. 2021;9[1]:45-49.
INTRODUCTION
Mycosis fungoides (MF) and Sézary syndrome (SS), two of the most-studied types of cutaneous T-cell lymphoma (CTCL), account for approximately 60% and 10% of CTCL cases, respectively.1 EMJ sat down with Richard Cowan, an oncologist with The Christie NHS Foundation Trust, University of Manchester, Manchester, UK, with expertise in CTCL, to discuss MF and SS diagnoses and treatments, their impact on patients, and unmet needs.
PRESENTATION
CTCL is a rare type of non-Hodgkin lymphoma that presents in the skin, with a subset of patients presenting with or developing extracutaneous disease.1 MF may appear as patches, plaques, and tumours or a mixture of these, and rarely with erythroderma. Lesions are usually confined to ‘bathing trunk areas’ under the arms and on the upper legs, with progression also occurring from one area to another.2 In SS, there is widespread erythroderma and often lymphadenopathy.2 CTCL has an incidence of approximately 1/120,000 people, as found in one USA study;3 it most commonly starts in a person’s mid-50s, and predominates in men.4 While CTCL can be fatal, with survival times under 5 years for SS and advanced MF, for those with early-stage MF, life span may be normal.5
In early MF especially, the clinical appearance may be misdiagnosed as eczema, psoriasis, or a fungal infection, leading to a median diagnostic delay of 36 months.4 CTCL can also be difficult to diagnose histopathologically, explained Cowan, as lymphocyte infiltration in biopsied skin can be seen in many inflammatory conditions. Cell surface receptor expression analysis can reveal CD4+/CD7- and/or CD4+/CD26- neoplastic cells.6 Cellular atypia and the characteristic distribution of these cells such as epidermotropism or tagging can be helpful in diagnosis. Cell surface expression analysis can reveal loss of certain T-cell antigens such as CD5 and CD7; such changes are indicative but not diagnostic.
Blood involvement may affect up to one in five early-stage patients and has been linked with worse outcomes and a higher risk of disease progression.4,7 Therefore, quantification of blood tumour burden is important and should be used for staging, tracking therapeutic response, and monitoring for disease progression:8 B0 (<250 cells/mL) signifies no significant blood involvement; B1 (≥250 and <1,000 cells/mL) is classified as low blood tumour burden; and B2 (≥1,000 cells/mL) indicates a high blood tumour burden. B2 is synonymous with SS, which can alternatively be diagnosed by detection of a CD4:CD8 ratio ≥10 and circulating T-cell clones with cerebriform nuclei.6,9
THE IMPACT OF CUTANEOUS T-CELL LYMPHOMA
“We must stop and think what [CTCL] must be like day in, day out to realise the impact,” Cowan stressed. Symptoms “set patients aside; they’re either suffering physically or socially, and emotionally they’re being distanced from ‘normal’ people.” This has been confirmed in several studies.10-12 Cowan highlighted how CTCL impact can occur at any stage: “We might see some in whom we consider the disease quite mild but we mustn’t underestimate this: those patients too are suffering emotionally, [they are] self-conscious, embarrassed, and annoyed they can’t get rid of this disease.”
The extreme itchiness of CTCL, Cowan emphasised, “can be absolutely debilitating, interfering with sleep, work, and day-to-day life. We fail our patients miserably,” he continued, “in that there is no good treatment for itch apart from treating the underlying condition.” Regarding SS, Cowan discussed how additional problems include being very conscious of the bright red erythroderma, the distressing occurrence of continually flaking skin, and difficulties in maintaining core body temperature due to inflamed skin “emanating vast amounts of heat.”
DIAGNOSIS
“GPs [general practitioners] rarely see CTCL,” reported Cowan, and diagnostic delay may be both through misdiagnosis and because CTCL can initially respond to topical corticosteroids in a manner similar to more-common skin conditions.2 It is usually only on progression that a dermatology referral occurs, although even general dermatologists and histopathologists may have little CTCL experience, explained Cowan.
A multidisciplinary team is needed for more complete diagnosis and management, comprising a CTCL-experienced dermatologist, a dermato- and haemato-pathologist experienced in skin and lymphoma, respectively, and an oncologist experienced in delivering CTCL-tailored treatments, discussed Cowan. He stressed the need for specialist nurses who have time to support patients and liaise with nursing care closer to home.
When it comes to making a diagnosis within this MDT, Cowan clarified: “Even with all the information, it’s not infrequent for us to say: ‘We’re not sure.’ It’s a distressing situation for the patient who’s come all the way to the specialist unit with the diagnosis of a malignant condition hanging over them and at the end of it all we say: ‘We’ll keep an eye on it’.”
TREATMENT
CTCL treatment usually starts with skin-directed therapies such as topical corticosteroids or a topical mustine when available.2,13 Twice-weekly narrow-band ultraviolet B phototherapy is the next option. This may be combined with oral psoralen, which Cowan reported can lead to a long-lasting response.2,9,13 In MF, persistent local disease leads to radiotherapy,2,9,13 to which CTLC lesions are “exquisitely sensitive,” highlighted Cowan, so very low doses can be used, minimising side effects. With more widespread disease, total skin electron-beam therapy can be employed delivering low dose radiotherapy to the whole skin surface.2,9,13
The next treatment step is systemic therapy, with, according to Cowan, no one therapy standing out as first line. Options here include pegylated α-interferon, low-dose methotrexate, and the rexinoid bexarotene.9 Immunotherapy is another systemic choice for more advanced disease, with the CD30-targeted agent brentuximab vedotin being efficacious in those with CD30+ CTCL.2 Mogamulizumab has shown advantageous results14 and is discussed further below. For SS, treatment can also include extracorporeal photopheresis administered on two consecutive days at specialised centres, repeated every 2 or 4 weeks.6,15
Advanced disease treatment can also involve chemotherapy, via CHOP (cyclophosphamide, doxorubicin, vincristine, oral prednisone), prolonged-release doxorubicin, or gemcitabine.6 However, Cowan noted that, while the response may be quick, it can be short-lived and require maintenance therapy. While CTCL is currently deemed incurable, remission has been achieved for some following allogeneic stem cell transplant (ASCT).13 Reduced-intensity ASCT may open this therapy to more patients;9 however, Cowan highlighted an unmet need to find treatments efficacious enough to bring CTCL under sufficient control to undergo ASCT, which necessitates a low tumour burden.
POST-HOC ANALYSIS OF THE MAVORIC TRIAL
Mogamulizumab is a humanised IgG1 κ monoclonal antibody that targets the CCR4 chemokine receptor prevalent on malignant T cells.14 Cowan was a principal investigator in the international Phase III MAVORIC trial, the largest-ever randomised trial of systemic therapies in CTCL, where significant advantages for relapsed/refractory MF or SS were shown with mogamulizumab (intravenous 1.0 mg/kg weekly for 28 days, then every 2 weeks) in progression-free survival and overall response rate compared to vorinostat (400 mg/day orally).14
Recent post-hoc analysis found mogamulizumab was significantly advantageous over vorinostat in those patients with B1 or B2 blood tumour burden for progression-free survival, overall response rate (B2 only), and time to next treatment (Figure 1). Treatment-related treatment-emergent adverse events were less frequent with mogamulizumab and were similar regardless of blood tumour burden.16
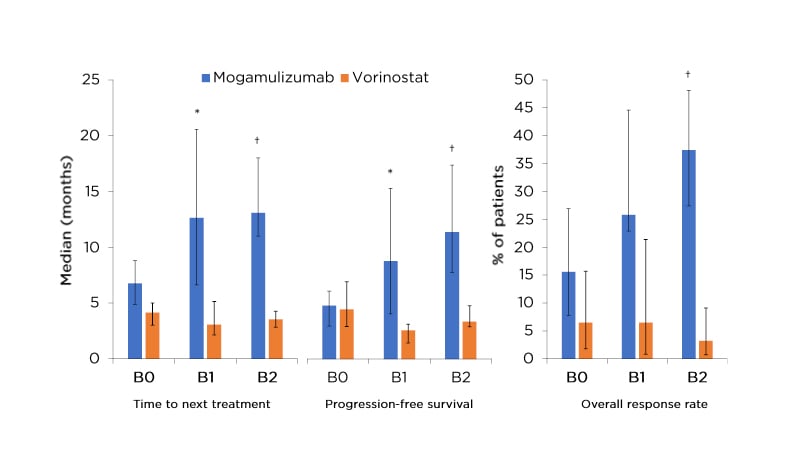
Figure 1: Results of post-hoc analysis of the MAVORIC trial.16
*p<0.05.
†p<0.0001.
95% confidence intervals indicated on graphs. Participant numbers for progression-free survival and overall response rate (and time to next treatment): B0: mogamulizumab, n=64 (n=49); vorinostat, n=62 (n=46); B1: mogamulizumab, n=31 (n=25); vorinostat, n=31 (n=18); B2: mogamulizumab, n=91 (n=82); vorinostat, n=93 (n=52).
Analysis of median percentage change in skin response over up to 12 treatment cycles revealed that both mogamulizumab and vorinostat led to progressive decreases.
Notably with mogamulizumab, B1 and B2 groups had the greatest improvements, suggesting a positive correlation between baseline blood involvement and mogamulizumab efficacy in skin.17
Similarly, investigation of health-related quality of life factors, including emotional, functional, pruritus, and symptom domains, again showed the largest differences in those with B1 or B2 blood tumour burden, with scores on one measure showing improvements only in this cohort who received mogamulizumab, from Cycle 3 onwards, with a decline in scores for those without blood involvement and those receiving vorinostat (B0, or B1 or B2).18
As recurrent haematogenous seeding of lesions has been proposed as one factor possibly driving MF progression,19 mogamulizumab may be exerting its effects by inhibition of this skin seeding, which could be the mechanism behind why it is particularly advantageous for patients with blood involvement.
MAVORIC notably contained a large number of patients with SS, for whom previously, explained Cowan: “Our ability to change the course of disease has remained frustratingly static.” He continued: “Suddenly, there’s a drug that has dramatically changed the picture.” He discussed that with mogamulizumab “we seem to be seeing an enhanced response in patients who have the most aggressive, poor-prognosis type of disease;” that is, those with the highest blood involvement.
THE UNMET NEEDS OF PEOPLE WITH CTCL
Cowan highlighted how “the great emphasis on future research…is looking at ways in producing agents that have a longer duration of response or finding approaches by which we can maintain the response, be it with milder agents or immune modulation.” He also discussed a large unmet need for therapies to be developed that are easier for patients to access (e.g., to address the disruption of visiting a phototherapy centre twice per week for many weeks). Further unmet needs include multi-compartmental efficacy and drug tolerability.
As CTCL is rare, there are little data to determine the treatment impact on early-stage disease. This unmet need is poised to be fulfilled by the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study, which involves an international database where patient data are collected in a unified fashion, including clinical, histopathological, and blood details alongside quality-of-life indices.4 Data are added annually and at every new therapeutic intervention. Cowan explained how, with nearly 1,500 patients already, “we will start to identify prognostic factors that will help guide us into the therapeutic approaches of the future.”
Finally, as patient organisations can support patients and families in better understanding CTCL and its management, Cowan discussed the importance of establishing national and international patient support groups, as patients with CTCL may feel especially isolated as “literally nobody’s ever heard of their disease.”







