Abstract
The treatment landscape in multiple myeloma has significantly changed since the introduction of high-dose melphalan with autologous stem cell rescue in the 1980s. Many randomised controlled trials have clearly demonstrated the superiority of autologous stem cell transplantation in improving survival compared to conventional chemotherapy. However, outcomes in myeloma are highly variable with median survival as short as 2 years and as long as 10 years or more. The main adverse factor predicting shorter survival is presence of high-risk cytogenetics. However, there are many other potential factors that can contribute to the treatment outcomes. This review looks at the various pretransplant variables that are associated with post-transplant outcomes in myeloma.
INTRODUCTION
Multiple myeloma is a malignant neoplasm arising from clonal proliferation of plasma cells in the bone marrow.1 Active myeloma is defined by the presence of CRAB criteria (hypercalcaemia, renal dysfunction, anaemia, and bone lesions) and/or by the presence of biomarkers (plasma cells: ≥60%; involved-to-uninvolved serum free light chain ratio: ≥100; >1 focal lesion on MRI).2 Despite so many advances in the field of myeloma and the availability of many newer potent agents, myeloma remains incurable. However, these newer agents have undoubtedly helped improve survival in myeloma to the extent that it can now be considered a chronic condition in some patients.3,4
The treatment landscape in myeloma has significantly changed ever since the introduction of high-dose melphalan with autologous stem cell rescue in the 1980s. The first randomised controlled trial (RCT) by the Intergroupe Francophone du Myélome (IFM) in 1996 clearly showed the superiority of high-dose melphalan followed by autologous stem cell transplantation (ASCT) over conventional chemotherapy.5 Since then, high-dose melphalan with stem cell transplantation has been the backbone of myeloma treatment. However, outcomes in myeloma are highly variable with median survival as short as 2 years and as long as 10 years or more.6 The main risk factor associated with shorter survival is presence of high-risk cytogenetics.6 However, there are many other potential factors that can contribute to the treatment outcomes. Hence, this review explores the various pretransplant variables that are associated with post-transplant outcomes.
BENEFIT OF AUTOLOGOUS STEM CELL TRANSPLANTATION
The results from the first two landmark clinical trials, IFM 90 trial and UK Medical Research Council (MRC) trial, have proven the superior role of ASCT in improving progression-free survival (PFS) and overall survival (OS) compared to conventional treatment. In the IFM 90 trial, involving 200 patients, ASCT was associated with higher complete responses (CR) (22% versus 5%), 5-year event-free survival (EFS) (28% versus 10%), and higher OS (52% versus 12%).5 In the MRC trial, involving 401 patients, results were similar to IFM 90 with improved CR, PFS, and OS in the ASCT arm.7 Three later RCT, however, failed to show any survival benefit of ASCT.8-10 Differential benefits on survival from these trials have to be addressed with caution as there was variability in the patients’ age groups, induction and conditioning regimens, and the response criteria. The role of ASCT in the era of modern drugs, such as proteasome inhibitors (PI) and immunomodulatory imide drugs, was studied in four RCT, and had shown benefit in favour of ASCT in improving PFS in all four and OS in two.11-14 In the largest study, involving 1,503 patients from multiple centres under the European Myeloma Network (EMN) group, ASCT improved response rates (≥very good partial response [VGPR] 84% versus 77%, and median PFS [57 months versus 42 months]), however, there was no OS benefit.14 A meta-analysis of the aforementioned mentioned trials using novel drugs, also concluded that there was significant improvement in PFS and a trend for benefit in terms of OS (hazard ratio for PFS: 0.55; p<0.001; hazard ratio for OS: 0.76; p=0.20).15 The main findings from the key RCT are shown in Table 1.
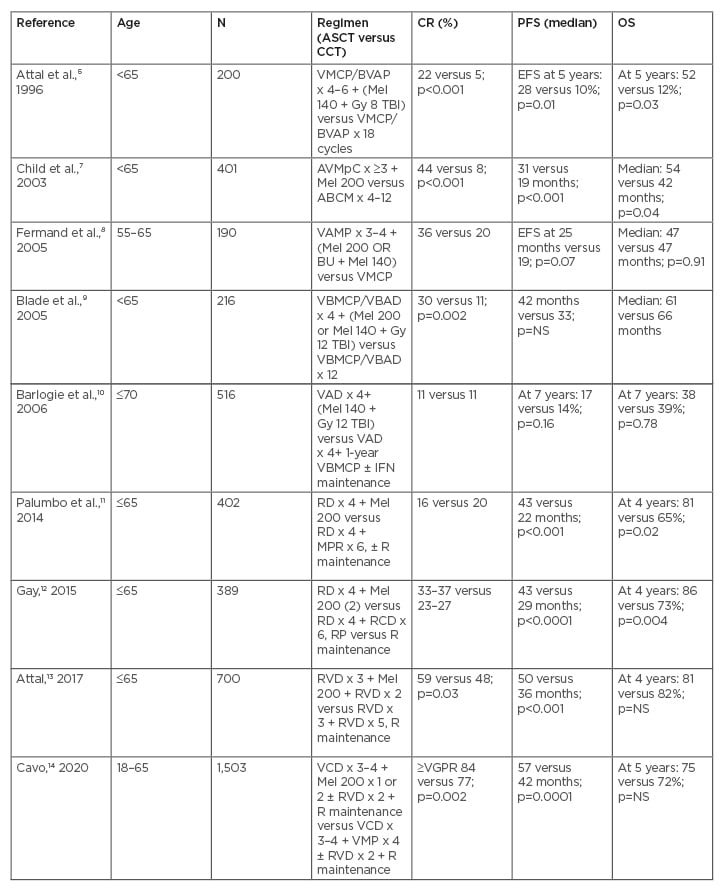
Table 1: Key randomised controlled trials comparing autologous stem cell transplantation with conventional chemotherapy in myeloma.
ABCM: adriamycin/carmustine/cyclophosphamide/melphalan; ASCT: autologous stem cell transplantation; AVMpC: adriamycin/vincristine/methylprednisolone/cyclophosphamide; BU: busulfan; CCT: conventional chemotherapy; CR: complete response; EFS: event free survival; IFN: interferon; Mel 140: melphalan 140 mg/m2; Mel 200: melphalan 200 mg/m2; MPR: melphalan/prednisolone/lenalidomide; NS: not significant; OS: overall survival; PFS: progression free survival; R: lenalidomide; RCD: lenalidomide/cyclophosphamide/dexamethasone; RD: lenalidomide/dexamethasone; RP: lenalidomide/prednisolone; RVD: lenalidomide/bortezomib/dexamethasone; TBI: total body irradiation; VAD: vincristine/adriamycin/dexamethasone; VAMP: vincristine/adriamycin/methylprednisolone; VBMCP: vincristine/carmustine/melphalan/cyclophosphamide/prednisolone; VBMCP/VBAD: vincristine, carmustine, melphalan, cyclophosphamide, prednisolone/vincristine, carmustine, adriamycin, dexamethasone; VCD: bortezomib/cyclophosphamide/dexamethasone; VGPR: very good partial response; VMP: bortezomib/melphalan/prednisolone; VMPC: vincristine/melphalan/cyclophosphamide/prednisolone; VMCP/BVAP: vincristine, melphalan, cyclophosphamide, prednisolone/carmustine, vincristine, adriamycin, prednisolone.
Adriamycin® (Pfizer Inc., New York City, New York, USA).
CONVENTIONAL KARYOTYPING-BASED RISK ASSESSMENT
Conventional cytogenetics reveal abnormalities in roughly one-third of patients with myeloma.16,17 These are mainly numerical aberrations. The low yield is caused by the low proliferative nature of plasma cells. Monosomy or deletion (q) of chromosome 13 and hypodiploidy are considered as high-risk features and are associated with inferior survival. In a report from the Mayo Clinic, 290 patients received information on conventional karyotyping: 39% had undergone ASCT and the median survival time of patients with high-risk cytogenetic abnormalities (CA) was 29 months versus 65 months in those at standard risk (p=0.006).18 The Groupe Français de Cytogénétique Hématologique showed that among 208 patients, 75 patients had a hyperdiploidy karyotype and 63 were labelled as having hypodiploidy defined as pseudodiploid, hypodiploid, or near-tetraploid chromosomes. In a multivariate analysis, the hypodiploid group had inferior survival compared to hyperdiploid group and del 13q lost its prognostic value in presence of hypodiploidy.19 Chromosome 13 abnormality retains a negative prognostic value even in patients undergoing high-dose chemotherapy and autotransplant.20,21 However, it is to be noted that detection of del 13q by interphase fluorescent in situ hybridisation (FISH) does not have any negative impact on hyperdiploid myeloma.22
FISH-BASED RISK ASSESSMENT
As a result of the poor yield by conventional karyotyping, more sensitive measures such as FISH are routinely performed for myeloma prognostication. FISH, currently considered as the standard assay, is performed on purified CD138-expressing plasma cells, or by dual-staining of cytoplasmic immunoglobulin-aided FISH.6 The International Myeloma Working Group (IMWG) classifies myeloma as high-risk if at least one of t(4;14), t(14;16), t(14;20), or del 17p is detected by FISH.23 In addition, a gain of 1q also confers poor risk.24 Bortezomib-containing induction regimen combined with ASCT can overcome the poor prognostic impact of t(4;14) and to some extent del 17p. In the Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) trial, in which 25% of the patients had t(4;14), bortezomib/thalidomide/dexamethasone (VTD) treatment could negate the poor prognostic role of t(4;14) (3-year PFS of 69% in patients with t(4;14) versus 74% without; p=0.66), whereas the thalidomide/dexamethasone arm could not (3-year PFS of 37% in patients with t(4;14) versus 63% without; p=0.01).25 Data from 354 patients with myeloma from the HOVON-65/GMMG-HD4 trial showed that bortezomib could significantly reduce the adverse impact of del 17p, which was present in 11% of patients. Patients were randomly assigned to cycles of vincristine/adriamycin® ([doxorubicin] Pfizer Inc., New York City, New York, USA)/dexamethasone (VAD) or bortezomib/adriamycin/dexamethasone induction followed by ASCT. In patients with del 17p, the bortezomib arm led to significant improvement in both PFS (median: 26 months versus 12 months) and 3-year OS (69% versus 17%).26 Combinations of bortezomib with lenalidomide/dexamethasone27,28 or carfilzomib with lenalidomide/dexamethasone29 are also effective in reducing the adverse impact of t(4;14) and/or del 17p on PFS in myeloma. Major CA and their impacts are summarised in Table 2.
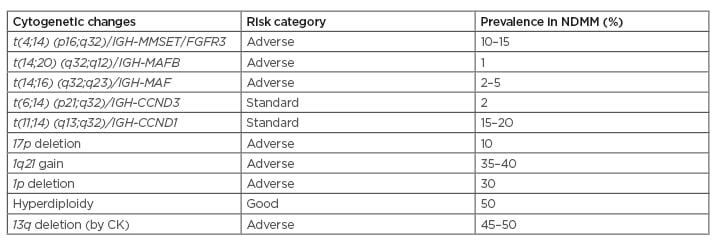
Table 2: Important cytogenetic alterations in multiple myeloma.
CK: conventional karyotyping; NDMM: newly diagnosed multiple myeloma.
THE REVISED INTERNATIONAL STAGING SYSTEM
The International Staging System (ISS) helps to stratify myeloma based on two parameters, albumin and β2-microglobulin, and categorises patients into three groups: ISS Stage I, II, and III, with median survival of 62, 44, and 29 months, respectively.30 The Revised-ISS (R-ISS) was formulated by combining ISS parameters with two additional parameters, namely a lactate dehydrogenase (LDH) assay and high-risk CA. It was derived from clinical and laboratory data, pooled from 11 international trials involving 4,445 newly diagnosed patients with myeloma. R-ISS stage I was defined as ISS stage I (β2-microglobulin <3.5 mg/L and albumin >3.5 g/dL), no high-risk CA (del 17p, and/or t[4;14], and/or t[14;16]), and normal LDH.
R-ISS stage III was defined as ISS stage III (β2-microglobulin >5.5 mg/L) and high-risk CA or high LDH. R-ISS could retain its value in delineating patients who had also undergone ASCT into the three risk groups. In total, 60% of patients had undergone ASCT and the median OS in R-ISS I, II, and III groups were ‘not reached’, 88 months, and 42 months, respectively.31
TYPE OF INDUCTION CHEMOTHERAPY
Induction chemotherapy in transplant-eligible patients is given with the purpose of improving symptoms, performance status, regaining normal renal functions, correcting hypercalcaemia, and achieving an optimum response. Before the availability of novel agents, VAD was the main induction regimen for myeloma. Response rates were less, in the range 50–55%, and with very few patients achieving CR.32 Toxicity related to anthracyclines, need for continuous infusion, and the risks associated with central venous catheters were the major concerns with this regimen. Later on, efforts were made to replace doxorubicin with a liposomal form, which led to similar response rates and survival, with less toxicity.33
After the introduction of novel agents, many studies have proven superiority to the same degree. Combination of thalidomide with dexamethasone improved response rates, both VGPR and CR, compared to VAD, before transplant.34,35 A meta-analysis of over 6,000 transplant-eligible patients with myeloma also reported the superiority of novel agents in improving PFS compared to the VAD regimen.36 Three-drug induction regimens are more effective than two-drug regimens in improving response rates and PFS. In two RCT and one meta-analysis, VTD improved response rates and PFS compared to thalidomide/dexamethasone.25,36,37 In a Phase III trial comparing VTD with bortezomib/dexamethasone (VD), the former led to better response rates; however, there were no differences in PFS or OS, which could be explained by the higher numbers of patients receiving consolidation and maintenance therapy in the VD arm.38 In the Southwest Oncology Group (SWOG) S0777 trial, combination of lenalidomide with VD (RVD) was compared against lenalidomide/dexamethasone, and the RVD arm showed improved response rates, PFS, and OS.39 In one of the largest retrospective studies, comparing different induction regimens, RVD led to superior response rates and OS relative to bortezomib/cyclophosphamide/dexamethasone and VD.40
Since the introduction of next-generation PI, namely carfilzomib, which has been reported to induce very high rates of minimal/measurable residual disease (MRD) negativity,41 there has been a growing enthusiasm for substituting bortezomib with carfilzomib in the induction regimens. However, two recently published Phase III trials failed to demonstrate any advantage of carfilzomib over bortezomib with respect to response, PFS, or OS. In the ENDURANCE trial, 1,087 patients newly diagnosed with myeloma, without high-risk features, were randomised to nine cycles of induction with either carfilzomib/lenalidomide/dexamethasone or bortezomib/lenalidomide/dexamethasone, followed by another randomisation to lenalidomide maintenance for 2 years versus until progression or toxicity. Median PFS (35 months versus 34 months) and 3-year OS (86% versus 84%) were similar between the two groups.42 In another Phase III trial (CLARION), in elderly patients with myeloma, similar results were obtained when patients were randomly assigned to bortezomib/melphalan/prednisolone versus carfilzomib/melphalan/prednisolone.43 Recently, combinations of daratumumab to triple-drug induction regimens have been trialled; daratumumab was added to bortezomib/cyclophosphamide/dexamethasone, bortezomib/lenalidomide/dexamethasone, bortezomib/thalidomide/dexamethasone, and carfilzomib/lenalidomide/dexamethasone in various trials and resulted in encouraging response rates with acceptable toxicity profiles.44
RESPONSE TO CHEMOTHERAPY
Many published studies have reported that stronger responses are associated with better survival in myeloma.45 This is also applicable to responses at the end of induction therapy in patients undergoing ASCT. In a single-centre study from the UK involving 383 transplant-eligible patients with newly diagnosed multiple myeloma, achievement of partial response or CR at the end of induction therapy led to improvement in OS (median: 7.47 years in responders versus 4.89 years in nonresponders).46 In another single-centre study involving 211 patients, CR at transplant led to better EFS (median EFS not reached in patients with CR versus 11 months in patients with less than CR).47 At least three more studies have shown that achieving CR or PR pretransplant was associated with improvement in both EFS/PFS and OS.48–50 These findings were further supported by a large meta-analysis of 21 ASCT studies involving nearly 5,000 patients, which showed that pretransplant maximum response (CR, near-CR, and VGPR) significantly improved both EFS/PFS and OS.51 A reduction in post-treatment fluorodeoxyglucose avidity scores by positron emission tomography was associated with improved survival outcomes. A Deauville score value of <4 of the bone marrow and focal lesions after therapy at premaintenance phase was associated with significant improvement in PFS and OS.52
There is a recent growing interest in outcomes based on MRD negativity. In the MRC Myeloma IX trial, end of induction MRD negativity with multiparameter flow cytometry significantly improved PFS post-transplant, but there was no difference in OS.53 The GEM/PETHEMA study group reported that pretransplant MRD negativity by multiparameter flow cytometry resulted in a significant improvement in PFS (5-year PFS: 80% in MRD negative group versus 25% in MRD positive group; p=0.001) and a trend toward improved OS (5-year OS: 100% versus 59%; p=0.06).54 A PCR-based MRD assessment study, from marrow samples, demonstrated that low levels of MRD pretransplant is associated with an improvement in both EFS and OS. Median EFS (35 months versus 20 months; p=0.001) and OS (70 months versus 45 months; p=0.04) were significantly better in the low MRD group versus the high MRD group.55 A meta-analysis of 21 studies, which measured response based on MRD criteria, reported that achieving MRD negativity could improve PFS and OS.56
TIMING OF TRANSPLANT
With the availability of many highly active novel agents for myeloma treatment, the role of transplant upfront was questioned. However, in an era of both conventional and newer agents, it has been demonstrated that early transplant improves response rates and PFS. An RCT, in the era of conventional agents, compared upfront transplant (early transplant group) against conventional chemotherapy (late transplant group). A rescue transplant was considered in the late group with progression/poor response to chemotherapy. The early group showed a better response and PFS, but with no difference to OS.57 Later, two retrospective studies, in the era of novel agents, failed to show any benefit of early transplant with respect to either PFS or OS.58,59 Gay et al.,60 in a pooled analysis of two RCT (RV-MM-209 and EMN-441), reported that early ASCT was associated with significant improvement in PFS1 (median: 42 months versus 24 months; p<0.001), PFS2 (4 years: 71% versus 54%; p<0.001), and OS (4 years: 84% versus 70%; p<0.001), and that the benefit was seen across all prognostic subgroups.60 In the only RCT using both lenalidomide and bortezomib in induction (IFM 2009), early transplant resulted in improved response rates (59% versus 48%), including MRD negativity, and better PFS (median 50 months versus 36 months), yet no difference in OS (at 4 years: 81% versus 82%).13 Despite the lack of definite OS benefit in the majority of studies, early transplantation should still be the first choice in multiple myeloma. Moreover, given the idea that achieving the maximum possible response, even to the extent of MRD negativity, can guarantee long-term survival benefit, early transplant should be offered in all possible situations.56 Transplant at progression, even though a reasonable choice, may be hindered because of concerns over advancing age, newly acquired comorbidities, and poorer response to salvage treatment.
In general, stem cell mobilisation is recommended after 4–6 cycles of induction chemotherapy. For mobilisation, two types of regimens are commonly used: granulocyte colony-stimulating factor alone or a combination of granulocyte colony-stimulating factor with cyclophosphamide. In case of inadequate mobilisation, plerixafor can be added. The specific mechanism of action of the latter, CXCR4 antagonism, helps to overcome the poor stem cell yield, associated with prolonged lenalidomide use in induction chemotherapy. Given the ongoing global coronavirus disease (COVID-19) pandemic, various national and international societies have recommended postponing autologous transplantation, especially in patients of standard risk.61,62
CONDITIONING REGIMENS
Many different conditioning regimens have been tried in myeloma over the last 20–25 years. Based on results from two prospective RCT, melphalan 200 mg/m2 (Mel 200) is now the accepted standard regimen. In the IFM 9502 trial, involving 282 patients <65 years of age, Mel 200 was compared with Mel 140 + 8 Gy total body irradiation. Mel 200 was associated with faster haematologic recovery, lower degree of mucositis, shorter duration of hospitalisation, and better OS at 45 months (66% versus 45%), even though there was no difference in EFS.63 In an Italian multicentre study, Mel 200 was compared against Mel 100; the study concluded that Mel 200 resulted in improved PFS (median: 31 months versus 26 months; p=0.01) and a trend toward improved OS (at 5 years: 62 months versus 48 months; p=0.13).64 Efforts to further intensify the conditioning regimen by additional chemotherapy did not show any benefit. In an RCT by the West German Myeloma Study Group, addition of idarubicin and cyclophosphamide to Mel 200 resulted in increased toxicity, high mortality, and no significant difference in response rates or survival.65 Similarly, addition of busulfan to melphalan,66,67 or a regimen containing thiotepa, busulfan, and cyclophosphamide (compared against Mel 200) did not result in superior outcomes.68 Addition of bortezomib or carmustine with high-dose melphalan has been investigated in Phase II studies and show promising response rates; however, larger RCT are required for confirmation.69,70
LIMITATIONS
Since this review has been written mainly from a practical point of view applicable to developing countries, where access to molecules like daratumumab is difficult and MRD measurement is not yet a routine practice, such things were not discussed in much detail. There is now strong evidence to suggest that achieving MRD negativity improves long-term survival and can be used as a surrogate endpoint for both PFS and OS in myeloma.71,72 Similarly, inclusion of anti-CD38 antibodies in the initial treatment regimen has improved PFS and OS in at least two RCT.73 The author supports these changes in response assessment and treatment becoming part of routine management for patients with myeloma, including in developing countries.
CONCLUSION
High-dose melphalan followed by ASCT is the cornerstone of treatment in medically fit patients with newly diagnosed myeloma and holds value even in the era of highly active, novel agents. There are important pretransplant variables that have a role in predicting post-transplant outcomes. CA by conventional cytogenetics (del 13q, hypodiploidy) or interphase FISH (del 17p, t[4;14], t[14;16], t[14;20]) are associated with inferior outcomes. Triple-drug induction regimens, containing a PI and immunomodulatory imide drugs, lead to the best outcomes post-transplant with respect to response rates and survival, with this particular combination negating the adverse impact of high-risk cytogenetics to a great extent. It is ideal to have the best response at the end of induction chemotherapy, which has been found to prolong survival after transplant. An early transplant rather than at the time of relapse/progression would be the ideal strategy, with Mel 200 as the standard conditioning regimen. Well-conducted clinical trials, especially RCT, should be considered for assessing the utility of MRD measurements in making treatment decisions, as MRD appears to be a powerful prognostic marker in myeloma.







