Abstract
Pleural effusion is a common adverse effect of dasatinib, but chylous effusion is rarely reported. Herein, the authors report the case of a 21-year-old imatinib-resistant patient who presented with bilateral massive chylous effusion on Month 44 of dasatinib treatment. The patient was managed with dasatinib withdrawal, bilateral thorax tube insertion, nasal oxygen support, diuretics, corticosteroids, a fat and oil free diet, and sandostatin. The patient required total parenteral nutrition and albumin infusion. The patient’s right lung collapsed as a result of pleural thickening. A subsequent switch to nilotinib was well tolerated. The authors highlight that patients on dasatinib treatment must be carefully followed for adverse effects.
INTRODUCTION
Dasatinib is a potent and efficacious second-generation oral tyrosine kinase inhibitor, frequently used for imatinib-resistant or intolerant BCR–ABL+ chronic myeloid leukaemia (CML) and for Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukaemia (ALL).1 Pleural effusion (PE) is a common adverse effect of dasatinib with a reported frequency of 14–32% in the published clinical trials.2-5 Pulmonary hypertension and parenchymal opacities are also reported adverse effects of dasatinib therapy, but dasatinib-related chylothorax is quite rare.1
CASE REPORT
A 21-year-old female patient with chronic phase CML presented with a 3-week history of dyspnoea, cough, and oedema. The patient had received dasatinib treatment for 44 months.
Initial presentation with CML was at the age of 17 years, with malaise and splenomegaly. A complete blood count revealed haemoglobin levels of 9.5 g/dL, white blood cell count of 850,000/μL, platelet number of 180,000/μL, and a peripheral smear that showed 16% promyelocytes, 21% metamyelocytes, 38% band forms, 17% neutrophils, 6% lymphocytes, 1% monocytes, and 1% basophils. A bone marrow smear revealed 20% promyelocytes, 13% metamyelocytes, 43% band forms, 10% neutrophils, 6% lymphocytes, 3% monocytes, 1% basophils, 1% erythroblasts, 2% normoblasts, and 1% blasts. Too few metaphase samples were obtained to perform conventional cytogentic analysis at diagnosis, but molecular diagnosis revealed a BCR/ABL 210-kDa protein (p210) international score (IS) of 555.9664% and showed 90% positive BCR/ABL fusion with fluorescence in situ hybridisation (FISH). After cytoreductive treatment with hydroxyurea, 400 mg/day imatinib treatment was started. Haematologic remission was achieved at 3 weeks, partial cytogenetic remission was achieved at 3 months, but no complete cytogenetic response or molecular response by Month 12 of treatment (FISH BCR/ABL p210 11%). Dasatinib 100 mg/day treatment began on the 13th month after diagnosis. Complete cytogenetic remission was achieved by Month 17, but a major molecular response was not achieved (BCR/ABL p210 IS 0.38%). The patient tolerated dasatinib well until Month 44.
Physical examination of the patient found, bilateral respiratory sounds were diminished. Thorax X-ray revealed bilateral pleural effusion (Figure 1A). Diagnostic thoracentesis showed chylothorax (pleural triglycerides 1133 mg/dL and serum triglycerides 79 mg/dL) (Table I); as a result, dasatinib was stopped at once, a left thorax drainage tube was inserted, which drained 2,500 mL of chylous fluid, and, following the drainage of the chylous fluid, nasal oxygen was administered. Thorax and abdominal CT showed no lymphadenopathy, tumour mass, or any evidence for tuberculosis and ductus thoracic injury (Figure 1B). An oral and oil free diet, total parenteral nutrition, and albumin were administered due to hypoalbuminaemia. Methyl prednisolone 1 mg/kg/day was administered in three doses and furosemide put on the same line please was started.
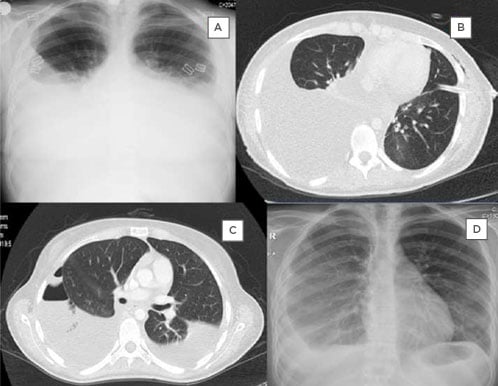
Figure 1: X-ray and CT images illustrating the progression pleural effusion exhibited by the patient and the interventions used by the authors.
A: Initial thorax X-ray showing bilateral pleural effusion. B: Thorax CT imaging after insertion of the left thorax tube. C: Thorax CT before insertion of the right thorax tube showing bilateral effusion and left pneumothorax. D: Thorax X-ray at 6-month follow-up showing collapse of the right lung due to pleural thickening.
On Day 4, drainage from the thorax tube was 500 mL/day. Octreotide 0.5 mcg/kg/hour was also administered in escalating doses but stopped on Day 9 due to no decrease in the chylous drainage.
At the end of the second week the patient had a febrile episode, but the fever subsided after piperacillin/tazobactam administration.
The tube was taken out after 20 days, the pleural liquid samples remained sterile, and no mycobacteria were detected. Methyl prednisolone and furosemide treatment was stopped at the end of the 4 weeks. The patient was discharged 28 days after initial admission with some pleural effusion in the right thorax. After 2 weeks she presented again with dyspnoea (Figure 1C) and a right thorax drainage tube was inserted. She was discharged with the thorax tube (100–200 mL drainage daily). After 2.5 months of dasatinib withdrawal, molecular genetics study of the peripheral blood showed BCR/ABL p210 IS of 2.83% (402 copies). Nilotinib was started (two 400 mg doses per day). After 3 months of chylous effusion, the right thorax tube was removed. There was no dyspnoea, but X-ray of the thorax showed partial collapse of the right lung due to pleural thickening (Figure 1D). After 5 and 8 months of nilotinib treatment, molecular genetics anlysis showed that BCR/ABL p210 IS was 0.19% (214 copies) and 0.38% (191 copies), respectively. Informed consent was obtained from the patient for publication of the paper.
DISCUSSION
The presented case details a 21-year-old female with chronic phase CML who received dasatinib 100 mg once per day for 44 months. It was reported that a single dasatinib dose of 140 mg per day was associated with significantly less PE compared with a 70 mg twice a day regimen (20% versus 39%; p<0.001) and a lower need to withdraw the drug.6 In other studies that compared 100 mg/day and 140 mg/day dasatinib treatment in single and two divided doses, no significant difference was found in the development of PE.7 In a study by de Lavallade et al.,8 a history of autoimmune disease and hypercholesterolaemia were factors associated with a higher risk of PE during dasatinib treatment.8 In reported patients, PE fluid was generally an exudate, and PE was neither related to fluid retention nor kidney failure or cardiac failure. The exact mechanism of PE remains unclear; the predominance of lymphocytes seen in most cases could indicate an immunological mechanism. Inhibition of the platelet-derived growth factor receptor beta (PDGFR-β) expressed in pericytes, which is involved in the regulation of angiogenesis, was also suggested.9 Src kinase inhibition by dasatinib was also possibly related to changes of vascular endothelial growth factor-mediated vascular permeability and stability of the pleural epithelium.10 The platelet-derived growth factor-signalling pathway stimulates tumour cell proliferation, angiogenesis, and pericyte recruitment to tumour blood vessels. Furthermore, ligated integrins recruit several nonreceptor tyrosine kinases, including focal adhesion kinase, integrin-linked kinase, and Src-family kinases, among others. If the presentation of Src kinases changes, it may lead to defects in integrin and further chylothorax.11 Chylothorax typically results from disruption of the normal lymphatic flow. Damage to the thoracic duct or its tributaries may cause leakage of the lymphatic fluid into the thoracic cavity. According to Light’s criteria, chylothorax is defined as a turbid PE with triglycerides >110 mg/dL.12 A visual inspection of the pleural fluid should be conducted, and milky pleural fluid should always be investigated for chylothorax. Not all chylothorax is exudative, with 20% of the cases being transudative.13 Generally, aetiology of the chylothorax is either traumatic or nontraumatic.14 The thoracic duct may be damaged by non-iatrogenic methods, such as subclavian vein catheterisation, fracture, dislocation of the spine, childbirth, and penetrating trauma from knife or gunshot injuries. Catheter-related venous thrombosis may also impair lymphatic drainage.15
Nontraumatic aetiologies include malignancy, sarcoidosis, retrosternal goiter, amyloidosis, superior vena cava thrombosis, benign tumours, congenital duct abnormalities, and diseases of the lymph vessels, such as yellow nail syndrome, lymphangioleiomyomatosis, and haemangiomatosis.14 Haematologic malignancies, including lymphoma, chronic lymphocytic leukaemia, and Waldenström macroglobulinaemia were reported to be associated with chylothorax.2 Imaging studies and history excluded such aetiologies. In the present case, analysis of the pleural fluid revealed chylothorax. Infection was not likely because the pleural fluid culture for tuberculosis and other bacteria remained sterile.
In , characteristics of the three reported patients with dasatinib-related chylothorax and the present case have been outlined.2-4 In the reported cases, management of the dasatinib-related PE involves the cessation of dasatinib therapy or reducing the dose and administration of diuretics and a short course of prednisone (40 mg daily for 4 days). Multicentre studies that enrolled many patients with dasatinib-associated PE, reported no cases with chylothorax.16,17 An Italian multicentre study investigated if dasatinib dose reduction after the first PE would prevent the recurrence of this adverse event. Dasatinib was temporarily interrupted in 71.9% of 196 cases, with a dose reduction in 59.2% of patients; however, recurrence was observed in 59.4% of the cases. Treatment was discontinued due to PE in 29.1% of the cases. Dasatinib dose reduction after the first episode of PE did not prevent recurrence of this adverse event. Therefore, the authors suggest that, once a major molecular response or a deep molecular response is achieved, different strategies of dasatinib dose management can be proposed prior to the development of PE, such as daily dose reduction or, as an alternative option, an on/off treatment with a weekend drug holiday.16 In a study by Hughes et al.,17 frequency, risk factors, and outcomes associated with PE were assessed in two Phase III trials (DASISION and 034/Dose-optimization) and a pooled population of 11 trials that evaluated patients with CML and Ph+ ALL treated with dasatinib. Pleural effusion developed in 6–9% of patients at risk annually in DASISION, and in 5–15% of patients at risk annually in 034/Dose-optimisation; with a minimum follow-up of 5 and 7 years, drug related PE occurred in 28% of 256 patients in DASISION and in 33% of 662 patients in 034/Dose-optimisation, respectively. A significant risk factor identified for developing PE, by a multivariate analysis, was advanced age.17 However, the age of the presented case is quite young.
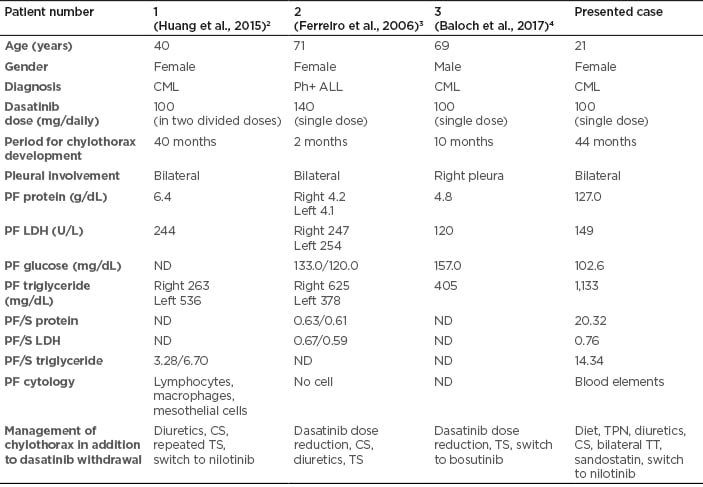
Table 1: Characteristics of patients with dasatinib-related chylothorax.
CML: chronic myeloid leukaemia; CS: corticosteroids; LDH: lactate dehydrogenase; ND: not defined; PF: pleural fluid; Ph+ ALL: Philadelphia chromosome-positive acute lymphoblastic leukaemia; S: serum; TPN: total parenteral nutrition, TS: thoracentesis; TT: thorax tube.
There are no firm guidelines detailing when to shift from one treatment to another. Due to respiratory distress, the authors conducted a thoracic tube insertion. The presented patient exhibited cyhlotorax and received 100 mg once-daily dasatinib. In the English literature reviewed by the author, seven patients with dasatinib-related chylothorax were reported.2-5,18 There was persistent chylous effusion in one patient which required 12 thoracentesis procedures.18
CONCLUSIONS
In the present case, administration of a reduced dasatinib dose was not trialed, due to life threatening chylotorax, with treatment shifted to nilotinib instead. At Month 10 of nilotinib therapy, the patient was in haematologic remission and her tolerance to the therapy was good, but respiratory function tests are still impaired.
Severe adverse events of dasatinib may be observed even during the fourth year of the therapeutic regimen. Dasatinib-related chylous effusion may be large and persistent; thus, shifting to another tyrosine kinase inhibitor may be required.







